Abstract
Mutations in PTEN activate the phosphoinositide 3-kinase (PI3K) signalling network, leading to many of the characteristic phenotypic changes of cancer. However, the primary effects of this gene on oncogenesis through control of the PI3K–AKT–mammalian target of rapamycin (mTOR) pathway might not be the only avenue by which PTEN affects tumour progression. PTEN has been shown to regulate the antiviral interferon network and thus alter how cancer cells communicate with and are targeted by immune cells. An active, T cell-infiltrated microenvironment is critical for immunotherapy success, which is also influenced by mutations in DNA damage repair pathways and the overall mutational burden of the tumour. As PTEN has a role in the maintenance of genomic integrity, it is likely that a loss of PTEN affects the immune response at two different levels and might therefore be instrumental in mediating failed responses to immunotherapy. In this review, we summarise findings that demonstrate how the loss of PTEN function elicits specific changes in the immune response in several types of cancer. We also discuss ongoing clinical trials that illustrate the potential utility of PTEN as a predictive biomarker for immune checkpoint blockade therapies.
Similar content being viewed by others
Background
Mutations in specific genes, including activating mutations in oncogenes and loss-of-function mutations in tumour-suppressor genes, are critical for cancer development.1,2,3 The inactivation or deletion of tumour-suppressor genes not only facilitates tumour development and progression, thereby keeping these cells in a continuous proliferative state,4 but might also influence immune responses.5,6 For instance, somatic inactivation or deletion of the gene that encodes the tumour-suppressor phosphatase and tensin homologue (PTEN)7 leads to the activation of phosphoinositide 3-kinase (PI3K) and subsequent downstream signalling to AKT and mammalian target of rapamycin (mTOR),8 a serine/threonine kinase that regulates cell growth, survival and proliferation.9 PTEN loss is also linked to aggressive cancer phenotypes.10 However, new studies have shown that, in addition to its established role in cancer progression, PTEN deficiency can lead to an immunosuppressive tumour microenvironment (TME)11,12,13,14 that is unfavourable for effective antitumour immune responses.15
Immune and tumour cells often communicate with each other by secretion of factors such as cytokines, chemokines, interleukins and extracellular vesicles.16 These signalling molecules can enhance or inhibit the activity of cytolytic cells, primarily represented by natural killer (NK) and CD8+ T cells.17,18 When CD8+ T cells directly recognise tumour antigens as non-self or are presented with tumour antigens through dendritic cells and macrophages, they target and kill the malignant cells.19 On the other hand, cytolytic cells are suppressed by cell-to-cell contact with regulatory T (Treg) cells and other immunosuppressive cells, and this is one of the several immune evasion mechanisms that cancers use to suppress antitumour immune responses.20
Immunotherapy does not target tumour cells directly: it acts by boosting the natural propensity of the immune system to recognise and respond to the presence of tumours.21 The principle of immune checkpoint inhibition involves the use of specific agents that block the suppressive interactions between a developing tumour and the defensive immune system of the patient.22 Immune checkpoint proteins such as programmed death ligand 1 (PD-L1), for example, downregulate the immune system and promote self-tolerance by suppressing T cell inflammatory activity against tumours, so that blocking the expression of checkpoint proteins with immune checkpoint inhibitors (ICIs) usually restores the capacity of the immune system to recognise tumour cells and kill them.23,24,25
Therapeutic response of individuals treated with ICIs is highly heterogeneous across tumour types, with some patients showing durable responses in previously incurable and highly aggressive cancers, but the majority of patients still have no appreciable benefit with these new drugs.26 Most mechanisms of resistance to ICIs involve functional changes occurring in the TME as well as the acquisition of intrinsic tumour-cell resistance.27 Tumour cell-intrinsic somatic mutations function at two different levels: not only do they promote higher levels of cell proliferation and cancer growth but they also activate mechanisms that allow tumours to avoid immune system attack.28,29 Recent findings have shown that the overall pattern of somatic mutations and genomic changes in tumours can be associated with resistance to immunotherapy in several different types of cancer,30,31 indicating that somatic point mutations, copy number alterations, epigenetic changes and dysregulation of gene expression can profoundly influence the interactions between immune and cancer cells.29,32 However, presently, the various molecular determinants of resistance to immunotherapy are complex and poorly characterised.
In this review, we discuss the most recent findings on PTEN in genomic stability, cancer immunogenicity, immune cell infiltration and the immune response across different cancers and summarise the emerging role of PTEN as a predictive biomarker for the use of ICIs. The techniques for detecting PTEN deficiency in tumours are now highly reproducible (see Table 1), which increases the potential utility of this biomarker for disease stratification33 and for predicting the chances of a successful response to immunotherapy.
The functions of PTEN
PTEN, one of the most commonly somatically mutated or deleted genes in cancer,7 is located at the 10q23.31 cytoband and consists of 9 exons that encode the full-length molecule of 403 amino acids.34,35
Cytoplasmic functions of PTEN
In the cytoplasm, the PTEN protein acts as a dual-specificity phosphatase and a direct antagonist of PI3K signalling by converting the second messenger phosphoinositol-(3,4,5)-trisphosphate into phosphoinositol-(4,5)-bisphosphate, which inhibits downstream signalling pathways36 that would otherwise normally mediate downstream activation of the AKT protein. Phospho-AKT activates different substrates, such as mTOR,37 a serine/threonine kinase that regulates cell growth, survival and proliferation. Somatic PTEN inactivation in tumours causes total loss of PTEN function that disrupts not only its catalytic phosphatase activities but also its regulatory control of the PI3K pathways. PTEN loss leads to downstream changes that govern important cellular processes crucial to cancer progression, including survival, proliferation, energy metabolism and changes in cellular architecture.
Nuclear functions of PTEN
In addition to its cytoplasmic functions in regulating cell growth and proliferation, PTEN regulates genome integrity and the stability of DNA repair in the nucleus.38 Pten-null mice show increased genomic and chromosomal instability, leading to centromere disruption, chromosomal translocations and spontaneous DNA double-stranded breaks that appear to occur independently of the PI3K–AKT–mTOR pathway. The protective role of PTEN in the genome is also supported by the finding that the PTEN.R189X mutation (which lacks the C-terminal region that is responsible for binding to the centromere protein CENP-C) leads to a significant increase in chromosomal aberrations, with transfected PTEN189 cells developing high levels of aneuploidy. This mechanism of protection is directly regulated by the interaction of PTEN with CENP-C, as well as by PTEN regulating the expression of Rad51 and its influence on the double-stranded break repair machinery.39 In glioblastoma, DNA repair is attenuated after cell exposure to ionising irradiation when nuclear PTEN is phosphorylated at position 240.40 The phosphorylated PTEN binds to chromatin and recruits RAD51 to promote DNA repair. In prostate cancer, PTEN also binds and promotes the degradation of the DNA-binding factor chromodomain helicase DNA-binding protein 1 (CHD1); PTEN deficiency leads to CHD1 protein stabilisation, which then engages the H3K4me3 epigenetic mark to activate transcription of downstream pro-tumorigenic and pro-inflammatory tumour necrosis factor α (TNFα)/nuclear factor κB (NF-κB) gene networks.41 NF-κB is a complex transcription system governing a diverse set of response genes mediating inflammatory and stress responses. The effects of PTEN on NF-κB transcription are also regulated by a translational variant of PTEN, called PTEN-L. The specific effects of loss of PTEN and PTEN-L on NF-κB-related immune responses are discussed below.
PTEN also regulates cellular senescence (i.e. irreversible arrest of cellular proliferation) mechanisms in cells that have lost proliferative capacity (reviewed in ref. 42). Loss of PTEN induces cellular senescence as a failsafe mechanism to defend against tumorigenesis.43 In prostate cancer, PTEN loss has been shown to activate p53-dependent cell senescence by mTOR kinase binding to p53.43,44 Concomitant loss of p53 allows cells to override the cytostatic effects of PTEN-induced senescence. Nuclear PTEN also interacts with the anaphase-promoting complex (APC) and regulates cellular senescence through APC–cadherin 1-mediated protein degradation.45 Moreover, senescence activation in PTEN-deficient cells is also associated with cytokine secretion that leads to an immunosuppressive TME.46
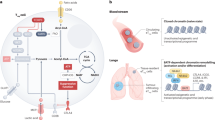
The various established PTEN signalling pathways associated with PI3K, genome stability and emerging features related to immune responses are summarised in Fig. 1.
Tumour-suppressor functions: The PI3K–AKT–mTOR pathway is negatively regulated by PTEN in the cytoplasm through the dephosphorylation of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) to phosphatidylinositol (4,5)-bisphosphate (PIP2). Increased activation of PI3K–AKT–mTOR leads to abnormal cell growth and proliferation. Cell senescence: PTEN also regulates cell senescence through the PI3K–AKT–mTOR network: the mTOR complex directly phosphorylates p53 that promotes the accumulation of p21. Consequently, p21 induces cell senescence. Nuclear PTEN also interacts with the anaphase-promoting complex (APC) and regulates cellular senescence through an APC–cadherin 1 complex. In this manner, PTEN loss promotes cell senescence as a failsafe against tumorigenesis. Immune and inflammatory response: PTEN negatively regulates the nuclear factor κB (NF-κB) signalling pathway through chromodomain helicase DNA-binding protein 1 (CHD1), which is ubiquitinated (Ub) in the presence of PTEN and thus is unable to promote the transcription of NF-κB genes in the nucleus. On the other hand, PTEN-L promotes the nuclear import of p65, which consequently induces the transcription of NF-κB genes. The presence of cytoplasmic DNA—as a consequence of genomic instability—activates the STING pathway, which phosphorylates interferon-regulatory factor 3 (IRF3). PTEN and PTEN-L are required for the migration of IRF3 into the nucleus, where this transcription factor mediates the immune response by promoting the expression of type I interferon (IFN) genes, such as interleukin (IL)-6 and chemokine (C-X-C motif) ligand 1 (CXCL1). DNA integrity: The concomitant presence of DNA damage repair (DDR) gene mutations or genome instability leads to double-stranded breaks (DSBs) in the DNA, which often causes self-DNA to migrate into the cytoplasm. Such genomic changes are also controlled by PTEN, since this tumour suppressor regulates cell cycle checkpoints, maintains centrosome stability and is involved in DNA repair.
PTEN inactivation, genomic instability and cancer immunogenicity
As outlined above, PTEN normally regulates several mechanisms that maintain genome stability in the nucleus, so PTEN-deficient cancers are known to exhibit high rates of chromosome rearrangements47,48 that are usually associated with increased mutational load.49 To better understand the consequences of aneuploidy in tumours, >10,000 tumours from The Cancer Genome Atlas were investigated in two studies.49,50 Both reports found that high levels of aneuploidy (defined as a whole chromosome or chromosome arm imbalances) were associated with decreased immune response levels, as determined by a low level of lymphocyte infiltration49 and a high expression of anti-inflammatory cytokines.50 However, this observation—that tumours with high aneuploidy levels are infiltrated by leukocytes to a lesser extent—seems to contradict the concept that increased levels of genomic change (higher mutational load) are often associated with increased immunogenicity.32 Tumours with increased mutational burden are more likely to produce neoantigens, which can be recognised as non-self and elicit an immune response. Increased immunogenicity may lead to a higher immune-cell abundance in the TME, improved patient survival and a better response to ICIs.51,52 As PTEN inactivation promotes higher rates of genomic instability,47 it would be generally expected that PTEN-deficient tumours would be pro-inflammatory, having a greater mutational burden and exhibiting increased immunogenicity in the TME. However, for many of the aneuploid tumours, it seems that immunoresistance may be able to suppress the inflammatory antigenic effects of higher mutational loads and increased genomic change49 (see Fig. 2).
PTEN loss is associated with cytokine and chemokine signalling that creates an immunosuppressive microenvironment. The TME becomes populated with immune cells that suppress the antitumour response, such as myeloid-derived suppressor cells (MDSCs), regulatory (Treg) cells and M2 macrophages (left side). PTEN-deficient tumours have also been linked with higher indoleamine 2,3-dioxygenase 1 (IDO1) and PD-L1 expression, which are known to reduce the activity of cytotoxic immune cells capable of killing cancer. In contrast, PTEN deficiency may also result in pro-inflammatory effects due to loss of the various nuclear functions of PTEN (right side). For example, there are higher levels of genomic instability, which may result in tumours that produce neoantigens. Neoantigens can elicit an immune response and activate CD8+ T cells. However, to counteract the effects of neoantigens, it is likely that tumours with high levels of genomic instability are able to suppress host immune responses to counter pro-inflammatory activity. TAM tumour-infiltrating macrophage.
Emerging evidence indicates that, in addition to growth-promoting PI3K alterations, PTEN loss is also associated with an immunosuppressive tumour state11,47,53,54,55 that favours cancer progression.53,54,56,57 For instance, in a murine model of T cell acute lymphoblastic leukaemia, Pten deficiency was reported to promote cell survival in a previously unsupportive TME.58 As discussed above, PTEN loss triggers senescence as a failsafe protection mechanism against cancer onset. Depending on the genetic changes already present in the tumour, PTEN-induced senescence can elicit changes in the cytokine network that result in an antitumour immune response in the TME46 (see Figs. 2 and 3).
Tumour cells harbouring PTEN loss are linked to a highly immunosuppressive environment mediated by myeloid-derived suppressor cells (MDSCs), regulatory (Treg) cells and M2 macrophages. In this model, the various changes caused by PTEN loss (shown schematically in Fig. 2) are thought to interact to suppress antitumour responses. There are likely to also be uncharacterised tumour-specific differences in immune response. For example, there is no consensus for the relationship between PTEN loss and CD8+ T cell density (for more details, see Table 2). TAM tumour-infiltrating macrophage, PD-L1 programmed death-ligand 1, IDO1 indoleamine-pyrrole 2,3-dioxygenase.
PTEN and the immune response
Several reports have shown that mutated oncogenes and tumour-suppressor genes have secondary functions in regulating the immune response in the TME in addition to their primary role in cell proliferation and survival in cancer (reviewed in ref. 5). In addition to its tumour-suppressor functions, PTEN normally influences the innate immune response by dephosphorylating Ser59 of interferon regulatory transcription factor 3 (IRF3) to enable its nuclear migration.13 Once in the nucleus, IRF3 promotes the transcription of type I interferon (IFN)-response genes.13 Type I IFN responses promote the expression of several genes that (i) control the response against infectious agents, (ii) modulate antigen presentation and cytokine expression and (iii) activate the T cell- and B cell-mediated immune response.60 Therefore, the activation of the type I IFN network is key in balancing the adaptive and innate immune responses. Both PTEN and PTEN-L have been shown to influence the transcription of type I IFN-regulated genes during viral infections by regulating IRF3 activation. PTEN-L regulates the import of IRF3 and the NF-κB component p65 into the nucleus, where both transcription factors activate type I IFN response and NF-κB-regulated genes, respectively.14 NF-κB regulates both immune and inflammatory responses and tumour-cell proliferation and apoptosis.61 PTEN-mediated enhancement of the type I IFN response is expected to promote the recruitment and activation of antitumour T cells and NK cells in addition to stimulating antigen presentation by dendritic cells.62 It is known that the hyperactivation of type I IFN responses is strongly linked to a better response to immunotherapy and increased activation of antigen presentation by tumour cells (reviewed in ref. 63). As a result, PTEN-deficient tumours might show impaired activation of both the type I IFN and the NF-κB pathways, which could be highly favourable for tumour progression because of an immunosuppressed TME.
The host antitumour immune response can also be triggered by cancer-specific genomic alterations and mutations.32 For instance, as a consequence of double-stranded DNA breaks caused by the presence of inactivating mutations in DNA damage repair (DDR) genes, there might be increased levels of DNA fragments in the cytoplasm of tumour cells.64 These increased levels of DNA fragments might activate the cytosolic double-stranded DNA-sensing cGAS-STING pathway, which promotes the phosphorylation of IRF3 (see Fig. 1), thereby promoting its retention in the cytoplasm. In this manner, PTEN deficiency in combination with DDR-mutated tumours might further attenuate the levels of immune response activation due to the lack of transcription of IFN-regulated genes by IRF3.65 Cao and colleagues14 showed that cells expressing only PTEN-L synthesised the highest levels of C-X-C motif chemokine ligand (CXCL) 10 and the pro-inflammatory cytokines interleukin (IL)-6 and CXCL1. In addition, Wang and colleagues66 demonstrated that the knockdown of PTEN-L significantly reduced the expression of TNFα and IL-6, which both have a pro-inflammatory role in mediating immune response. In this context, loss of PTEN and its secreted variant PTEN-L in tumour cells is likely to increase the activity of immunosuppressive cytokines on the immune cells in the TME. Interestingly, another three translational variants of PTEN exist; however, little is known about their effects in the immune response (reviewed in ref. 67). Thus the emerging role of PTEN in mediating the immune response is complex and involves multiple pathways that collectively repress antitumour responses in the TME (shown schematically in Figs. 2 and 3).
PTEN is also known to play a crucial role in the regulation of signalling in immune cells.12 Pten deletion of specific immune cell subsets in mice cause defects in T cells,68 Treg cells69 and B cells.70 Myeloid cell-specific PTEN deficiency and increased PI3K levels reduce inflammation, increase macrophage phagocytic ability and facilitate resistance to infection.71 These findings are consistent with the early observation that PTEN deficiency was associated with chronic inflammation and autoimmunity.72 Since inflammation and immunity are known key factors in tumour progression,73 it is important to understand how PTEN mediates responses in the TME.
PTEN deficiency and the immune response in cancer
The impact of PTEN loss and PI3K activity on T cell-mediated antitumour responses was investigated in a preclinical model of melanoma. Inhibition of PTEN-null tumours with a PI3K inhibitor led to in vitro killing by T cells and restoration of antitumour control of immune checkpoint inhibition using anti-PD-L1 and anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA4)30 antibodies. However, the authors demonstrated that PD-L1 and major histocompatibility complex class I expression do not constitute the primary mechanism of immunosuppression mediated by PTEN loss in melanoma. Their xenografting experiments suggested that the lack of response to ICIs could have resulted from a higher expression of vascular endothelial growth factor (VEGF) and increased secretion of inhibitory cytokines by tumour cells lacking PTEN.
PTEN loss was also found to be significantly associated with altered macrophage densities and CXCL8 expression in a study conducted using 28 radical prostatectomy-derived specimens.74 CXCL8 is a pro-inflammatory chemokine that binds to the C-X-C chemokine receptor type 1 CXCR1 and CXCR2. Together, the CXCL8–CXCR1/2 axis mediates tumour initiation and progression, in addition to promoting neovascularisation of the TME.75 Haploinsufficiency of Pten combined with oncogenic Kras mutations was associated with NF-κB pathway activation in a mouse model of pancreatic ductal adenocarcinoma. The authors of this study also showed that stimulation of the NF-κB network led to TME remodelling through the infiltration of immune cells with pro-tumorigenic properties.76
Immune checkpoint inhibition is most often met with little or no success in prostate cancer, owing to low levels of immune-cell infiltration. In Pten-null prostate conditional mice, tumours are strongly immunosuppressed as a result of the downregulation of the tyrosine-protein phosphatase PTPN11 and consequent Janus kinase 2 (Jak2)–signal transducer and activator of transcription 3 (Stat3) pathway activation.46 In another study conducted with Pten-deficient mice, additional ablation of the growth-regulatory kinase extracellular signal-regulated kinase 5 (ERK5) promoted T cell infiltration into prostate tumours through Ccl5 and Cxcl10 production.77 Thus the concomitant loss of ERK5 was able to restore immune functions caused by PTEN deficiency leading to tumours with higher T cell infiltration. These data provide important clues about why human prostate cancer does not always benefit from current immunotherapeutic approaches.
PTEN loss has also been linked to changes in the secretome (that is, cell-secreted molecules, such as chemokines and ILs) so that its role in mediating immune responses in the TME might also be indirect. In non-small cell lung cancer, CXCR4 expression was shown to be dependent on the PI3K–AKT–mTOR signalling pathway, which is directly controlled by PTEN.78 The loss of Pten in stromal fibroblasts of mouse mammary glands led to the altered production of cytokines and chemokines in the TME, increased macrophage recruitment and enhanced vascularisation, which together accelerated the initiation, progression and malignant transformation of mammary epithelial tumours.79 Another study conducted with murine prostate epithelial cells and human prostate cancer cell lines demonstrated that the loss of PTEN enhanced the expression of CXCR4 and CXCL12.80 Moreover, in prostate cancer cell lines and prostatic tissue, Maxwell and colleagues81 demonstrated that the expression and secretion of the pro-inflammatory chemokine CXCL8 were also associated with PTEN inactivation.
A recently published study conducted in mice demonstrated that the diversity of genomic changes in prostate cancer can determine the composition of immune cells within the TME.82 The authors showed that tumours derived from Trp53−/−Pten−/− tumours expressed high levels of Cxcl17, which recruited cells with immunophenotypes of monocytic myeloid-derived suppressor cells (MDSCs) and monocytes. Moreover, these tumours exhibited a Treg cell-mediated immunosuppressive signature that was linked to the tumour-promoting effect of MDSCs. The details of the role of MDSCs in PTEN-deficient tumours are discussed below. On the other hand, simultaneous ablation of Pten and Pml in mice was related to an ‘immune desert’ phenotype, characterised by low T cell densities and immune checkpoint expression. In summary, changes in Pten and the interplay with other genes studied in mouse models of prostate cancer have been shown to collectively shape the immune-cell content and expression of immunosuppressive markers in the TME.
PTEN also undergoes germline-inactivating mutations that are responsible for various human syndromes.83 The PTEN hamartoma tumour syndrome is a hereditary tumour syndrome that predisposes patients to benign and malignant breast, thyroid, renal cell and endometrial tumours.84 A recently published study demonstrated that the innate immune cells from patients with PTEN hamartoma tumour syndrome produce high levels of lactate.85 Lactate confers a pro-inflammatory phenotype of monocytes in the TME of PTEN-deficient thyroid cancer. Interestingly, when PTEN-deficient thyroid cancer cells were exposed to the immunomodulatory effects of metformin (which is used for treating Type 2 diabetes), there was a more pro-inflammatory phenotype in the TME.85 Such a phenotype reflects a more immunogenic TME, which presents increased infiltration of immune cells together with the synthesis of cytokines and chemokines. By contrast, treatment with rapamycin (an mTOR blocker) led to reduced pro-inflammatory cytokine expression. This study provided evidence that the PI3K–AKT–mTOR network directly influences the immune response within the TME of PTEN-deficient tumours. Indeed, mTOR activation directly promotes immune-cell differentiation (reviewed in ref. 86). It is thus critical to confirm the multiple roles of PTEN and its related signalling pathways in the control of the inflammatory and immune responses.
PTEN loss and immune cell infiltration
The levels of immunoreactive CD8+ T cells can be helpful in evaluating overall immune responses, but the immune-cell landscape of tumours that harbour PTEN somatic mutations is complex and highly heterogeneous, with some studies showing that PTEN deficiency is linked to a high CD8+ T cell density53,87 while other studies have shown the inverse correlation88,89,90 (Table 2).
A high degree of tumour infiltration by CD8+ T cells is strongly linked to a better prognosis in several types of cancer.91 It is known that immune-cell recruitment and activation relies on tissue-specific mechanisms, which vary across different tumour types92 and tissues, and might even present immune-cell contents that are distinct for different lesions from a single patient.93 Thus it is expected that PTEN deficiency in tumour cells could elicit effects on the TME that are tumour-type specific or even tissue specific. Interestingly, several reports have shown that tumours harbouring PTEN deficiency are more likely to present with an increased M2 macrophage cell density.22,94,95,96 The presence of M2 macrophages in tumours is linked to favourable growth and invasive tumour features through the synthesis of anti-inflammatory cytokines and the inhibition of antigen presentation and cytolytic cell activation and growth.97
Interestingly, PTEN-deficient tumours often exhibit high densities of Treg cells in the TME (Table 2). Treg cells have an immunosuppressive effect in the TME by inactivating the priming and effector activities of CD4+ and CD8+ T cells.59 Moreover, several reports have confirmed the associations between the density of Treg cells and poor outcome in different types of tumour.91 A recently published report on glioblastoma showed that PTEN deficiency was linked to high macrophage densities.22 Interestingly, the authors of this study found that the density of CD8+ T cells increased in PTEN wild-type tumours after PD1 blockade therapy. Conversely, PTEN-deficient tumours did not show a significant increase in CD8+ T cell density after therapy. Another new study on the impact of PTEN loss in glioblastoma demonstrated increased infiltration of macrophages via the yes-associated protein 1–lysyl oxidase b1 (LOX-b1)–integrin–PYK2 axis.98 The authors showed that LOX expression activated specific pathways in macrophages that promoted their recruitment into the TME where they secrete the growth factor osteopontin (SPP1).
Studies with mice have shown that PTEN deficiency can increase tumour-infiltrating MDSC densities in the TME (Table 2). MDSCs comprise a heterogeneous population of myeloid progenitors and immature granulocytes, macrophages and dendritic cells. Mechanistically, MDSCs are highly immunosuppressive cells that suppress CD8+ T cell activity through the secretion of reactive oxygen species, peroxynitrite and nitric oxide (reviewed in ref. 99). MDSCs are also thought to sustain tumour growth by protecting proliferating tumour cells from senescence.100 Owing to the ability of MDSCs to modulate the antitumour immune response, the presence of high densities of MDSCs correlates with a poor outcome in several tumours.91,101 In a preclinical prostate cancer, PTEN loss results in tumours with high expression of the cytokines colony-stimulating factor 1 and Il1b, which leads to an expansion of infiltrating MDSCs.57 The development of castration-resistant prostate cancer—a condition in which tumours are insensitive to androgen-deprivation therapy—was found to be associated with the presence of high densities of tumour-infiltrating MDSCs and high levels of IL-23, which can activate the androgen receptor pathway in prostate tumour cells to promote cell survival and proliferation.102 As PTEN loss occurs in 40–50% of castration-resistant prostate cancers, it is critical to understand how an immunosuppressive and tumour-tolerant TME is related to increased IL-23 and infiltration of MDSCs in such advanced cancers.
Although PTEN deficiency appears to shape the TME of cancers by mediating the secretion of signalling molecules, one can speculate that these effects could also be an indirect secondary consequence of PI3K–AKT pathway dysregulation. It is known that the PI3K pathway has a role in regulating the antitumour immune response and immune-cell differentiation.103 Reports on PI3K blockade indicate the associations between this signalling network and macrophages, CD8+ T cells and Treg cells (reviewed in ref. 104). However, PTEN inactivation is sometimes reported to occur together with downstream PIK3CA mutations.8 This finding suggests that some of the somatic mutations of the PTEN gene are redundant but are selected for because of the loss of other regulatory functions that are independent of its role in controlling PI3K activity.
PTEN deficiency and immune checkpoint expression
The most effective current immunotherapeutic interventions, such as PD-L1 inhibitors, are able to restore T cell activity against tumours.105 Unfortunately, some patients relapse after an initial response from immune-blockade therapies, suggesting that acquired tumour-specific alterations might trigger resistance to immunotherapies.5 As discussed above, the accumulation of somatic mutations (and thus high levels of neoantigens) and pathway dysregulation of tumours might lead to an altered immune cell composition in the TME that strongly influences therapy responses.105
As a result of innate immune resistance, the constitutive activation of oncogenic pathways can promote the synthesis of PD-L1 in tumour cells independently of the immune-cell state in the TME. For instance, in murine models of lung cancer, activation of the AKT–mTOR pathway promoted the expression of PD-L1.55 Functional studies with lung cancer cell lines also demonstrated that tumour-infiltrating macrophages might promote PD-L1 synthesis by secreting IFN-γ in a JAK–STAT3-dependent manner.106 Interestingly, PTEN-deficient melanomas and sarcomas express high levels of VEGFA and STAT3, while also showing resistance to ICIs.30,107 If PTEN loss occurs, the upregulation of angiogenesis, as mediated by VEGFA and STAT3, may also influence the trafficking of immune cells in the TME.108,109 It is known that the tumour blood vessels can be modulated by both inflammatory triggers and the types of infiltrating immune cell subsets in the TME. To infiltrate into the tumour and its TME, immune cells must enter the tumour vasculature, adhere to the endothelium and migrate across the blood vessel wall. For these reasons, there is interest in combining anti-angiogenic agents with ICIs to potentially improve immunotherapy responses (reviewed in ref. 110).
Several studies in breast cancer, colorectal cancer and glioma have shown that cancer cell intrinsic PD-L1 expression increases as a result of loss of PTEN. This finding suggests that the context of pre-existing tumour immune states could be informative for more precise use of immunotherapy (Table 2). Through in silico analyses of The Cancer Genome Atlas sarcoma specimens, it was shown that the presence of an oncogenic mutation and a heterozygous deletion in the remaining PTEN allele led to a lower expression of CD8+ T cell markers (granzyme A [GZMA], perforin 1 [PRF1], and cluster of differentiation 8A [CD8A]) and PD1 and interferon γ (IFNG) genes.107 This observation indicates that complete inactivation of PTEN is associated with a reduced CD8+ T cell density and activation within the sarcoma TME. In murine lung cancer models, activation of the PI3K–AKT–mTOR cascade resulting from PTEN deficiency was associated with the overexpression of PD-L1.111 This finding indicates that PTEN loss and consequent overactivation of the PI3K–AKT–mTOR induces PD-L1 expression. The authors also showed that tumour growth was inhibited, the number of tumour-infiltrating T cells was increased and the density of Treg cells decreased with the combined treatment of mTOR inhibition and anti-PD1 agents.
In PTEN-deficient breast cancer cell lines, Mittendorf and colleagues53 observed overexpression of PD1 and PD-L1, which was downregulated after PI3K pathway inhibition. Moreover, PD-L1 overexpression induced by PTEN loss in tumour cells was associated with low T cell proliferation rates.53 Similarly, the absence of PTEN expression in melanoma cell lines was associated with the upregulation of PD-L1 expression.112 In gliomas, PTEN loss was significantly associated with PD-L1 overexpression.54 PTEN mutations have been linked to anti-PD1 therapy resistance in glioblastomas.22 Indeed, PTEN-intact glioblastomas showed a high density of CD3+CD8+ cells after treatment with PD1 inhibitors, but PTEN-deficient tumours did not exhibit this effect.
In a murine model of castration-resistant prostate cancer, the combination of deletions of Pten, transformation-related protein 53 (Trp53) and Smad4 led to resistance to anti-PD1 and anti-CTLA4 antibodies.113 Interestingly, PTEN deficiency alone was not associated with PD-L1 expression in prostate cancer. Indeed, PD-L1 expression in radical prostatectomy-derived prostate tumours is rare.114 Collectively, these observations demonstrate that knowledge of the status of PTEN might be useful in deciding which patients and which specific tumour types might benefit from current immunotherapeutic approaches.
Clinical trials investigating the influence of PTEN on the response to immunotherapy
As PTEN functions determine how tumour cells react to the immune response in the TME, this gene might be a useful biomarker for determining how patients will respond to therapy. A Phase 2 trial showed that patients with PTEN-deficient castration-resistant prostate cancer had improved radiographic progression-free survival when treated with a combination of the hormone therapy drug abiraterone and an AKT blocker.115 Although PTEN biomarker testing has been a consideration for standard therapeutic responses, only a limited number of clinical trials plan to determine PTEN status in relation to the clinical response of tumours with ICIs (more details in Table 3). This suggests that PTEN might have been overlooked in trial designs, despite the compelling associations between this pathway and immunotherapy response. However, several downstream markers of the PTEN signalling pathway, such as AKT and PI3K, are being investigated in conjunction with checkpoint blockade therapies.
Interestingly, inhibition of components of the PI3K–AKT–mTOR network in PTEN-deficient primary cultures of gliomas led to a decrease in T cell death.116 The effects of restoration of PI3K control suggests that PTEN loss in tumours may be enhancing the adaptive immune response against cancer cells.117 Concordantly, ablation of the PI3K pathway led to a marked enhancement of the antitumour functions of Toll-like receptor ligands in different murine models of cancer.118 The outcome of several ongoing trials investigating genomic alterations and potential links between PTEN loss and response to ICIs (Table 3) could be very informative. The trials (NCT02299999, NCT02117167 and NCT03772561) will involve transcriptome analysis, so it will be possible to determine whether genomic changes such as PTEN loss, combined with other mutations, are involved in the differential response to immunotherapy. In this manner, determining PTEN deficiency in tumours might be useful for future biomarker-guided combination drug trials.
Concluding remarks
Various lines of evidence suggest that loss of PTEN has a crucial role in the development of an immunosuppressive cancer phenotype for some tumours. As restoring the function of PTEN is presently not feasible,119 the most obvious way to counter the effects of PTEN loss would be to inhibit PI3K signalling. However, as PTEN and PTEN-L appear to mediate immune responses independently of PI3K, future therapeutic approaches should target other downstream pathways and signalling molecules that directly control TME immune responses. For instance, blockade of the JAK–STAT3 pathway in PTEN-null prostate cancers induced the TME to become more immunogenic and to be infiltrated by increased numbers of T cells.46 In this way, tumours could be primed to respond to anti-PD1 or anti-PD-L1 therapy as the immune cells in the TME can be induced to better recognise tumour cells. Other approaches, such as anti-angiogenic therapies, might also benefit patients before exposure to immunotherapeutics.11 Interestingly, PTEN-deficient glioblastomas overexpressed the CD44 cell-surface adhesion receptor and had a more compact tumour-cell phenotype that could exclude vascularisation and immune cells from the TME than wild-type glioblastomas,22 rendering them less likely to respond to ICI. More studies are required to determine the mechanistic links between PTEN, angiogenesis and the response to anti-PD1/PD-L1. As many patients experience relapse after immunotherapy, it is critical to characterise new tumour-specific genomic biomarkers and the molecular signatures of response that will be informative for immune-based treatments.
References
Wang, P., Zhang, H., Yang, J., Li, Z., Wang, Y., Leng, X. et al. Mu–KRAS attenuates Hippo signaling pathway through PKCι to sustain the growth of pancreatic cancer. J. Cell. Physiol. 235, 408–420 (2020).
Cantley, L. C. & Neel, B. G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl Acad. Sci. 96, 4240–4245 (1999).
Adams, J. M., Harris, A. W., Pinkert, C. A., Corcoran, L. M., Alexander, W. S., Cory, S. et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318, 533–538 (1985).
Fisher, G. H. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 15, 3249–3262 (2001).
Wellenstein, M. D. & de Visser, K. E. Cancer-cell-intrinsic mechanisms shaping the tumor immune landscape. Immunity 48, 399–416 (2018).
Rooney, M. S. S., Shukla, S. A. A., Wu, C. J. J., Getz, G. & Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61 (2015).
Lee, Y.-R., Chen, M. & Pandolfi, P. P. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat. Rev. Mol. Cell Biol. 19, 547–562 (2018).
Millis, S. Z., Ikeda, S., Reddy, S., Gatalica, Z. & Kurzrock, R. Landscape of phosphatidylinositol-3-kinase pathway alterations across 19 784 diverse solid tumors. JAMA Oncol 2, 1565–1573 (2016).
Yehia, L., Ngeow, J. & Eng, C. PTEN-opathies: from biological insights to evidence-based precision medicine. J. Clin. Invest. 129, 452–464 (2019).
Nowak, D. G., Cho, H., Herzka, T., Watrud, K., DeMarco, D. V., Wang, V. M. Y. et al. MYC drives Pten/Trp53-deficient proliferation and metastasis due to IL6 secretion and AKT suppression via PHLPP2. Cancer Discov. 5, 636–651 (2015).
Cheng, F. & Eng, C. PTEN mutations trigger resistance to immunotherapy. Trends Mol. Med. 25, 461–463 (2019).
Chen, L. & Guo, D. The functions of tumor suppressor PTEN in innate and adaptive immunity. Cell Mol. Immunol. 14, 581–589 (2017).
Li, S., Zhu, M., Pan, R., Fang, T., Cao, Y.-Y., Chen, S. et al. The tumor suppressor PTEN has a critical role in antiviral innate immunity. Nat. Immunol. 17, 241–249 (2015).
Cao, Y., Wang, H., Yang, L., Zhang, Z., Li, C. C.-M., Yuan, X. et al. PTEN-L promotes type I interferon responses and antiviral immunity. Cell Mol. Immunol. 15, 48–57 (2018).
Horton, B. L., Williams, J. B., Cabanov, A., Spranger, S. & Gajewski, T. F. Intratumoral CD8 + T-cell apoptosis is a major component of T-cell dysfunction and impedes antitumor immunity. Cancer Immunol. Res. 6, 14–24 (2018).
Kryczek, I., Lange, A., Mottram, P., Alvarez, X., Cheng, P., Hogan, M. et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 65, 465–472 (2005).
Allen, F., Bobanga, I. D., Rauhe, P., Barkauskas, D., Teich, N., Tong, C. et al. CCL3 augments tumor rejection and enhances CD8 + T cell infiltration through NK and CD103 + dendritic cell recruitment via IFNγ. Oncoimmunology 7, 1–12 (2018).
Muthuswamy, R., Corman, J. M., Dahl, K., Chatta, G. S. & Kalinski, P. Functional reprogramming of human prostate cancer to promote local attraction of effector CD8 + T cells. Prostate 76, 1095–1105 (2016).
Segal, N. H., Parsons, D. W., Peggs, K. S., Velculescu, V., Kinzler, K. W., Vogelstein, B. et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 68, 889–892 (2008).
Tanaka, A. & Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 27, 109–118 (2017).
Tumeh, P. C., Harview, C. L., Yearley, J. H., Shintaku, I. P., Taylor, E. J. M., Robert, L. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Zhao, J., Chen, A. X., Gartrell, R. D., Silverman, A. M., Aparicio, L., Chu, T. et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 25, 462–469 (2019).
Koyama, S., Akbay, E. A., Li, Y. Y., Herter-Sprie, G. S., Buczkowski, K. A., Richards, W. G. et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 7, 10501–10511 (2016).
Deng, R., Fan, F., Yi, H., Liu, F., He, G., Sun, H. et al. PD-1 blockade potentially enhances adoptive cytotoxic T cell potency in a human acute myeloid leukaemia animal model. Hematology 23, 740–746 (2018).
Della Corte, C. M., Barra, G., Ciaramella, V., Di Liello, R., Vicidomini, G., Zappavigna, S. et al. Antitumor activity of dual blockade of PD-L1 and MEK in NSCLC patients derived three-dimensional spheroid cultures. J. Exp. Clin. Cancer Res. 38, 253–267 (2019).
Borcoman, E., Kanjanapan, Y., Champiat, S., Kato, S., Servois, V., Kurzrock, R. et al. Novel patterns of response under immunotherapy. Ann. Oncol. 30, 385–396 (2019).
Goodman, A. M., Kato, S., Bazhenova, L., Patel, S. P., Frampton, G. M., Miller, V. et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 16, 2598–2608 (2017).
Quigley, D., Silwal-Pandit, L., Dannenfelser, R., Langerød, A., Vollan, H. K. M., Vaske, C. et al. Lymphocyte invasion in IC10/basal-like breast tumors is associated with wild-type TP53. Mol. Cancer Res 13, 493–501 (2015).
Wang, Q., Hu, B., Hu, X., Kim, H., Squatrito, M., Scarpace, L. et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32, 42–56 (2017).
Peng, W., Chen, J. Q., Liu, C., Malu, S., Creasy, C., Tetzlaff, M. T. et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 6, 202–216 (2016).
Rizvi, N. A., Hellmann, M. D., Snyder, A., Kvistborg, P., Makarov, V., Havel, J. J. et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 348, 124–128 (2015).
Thorsson, V., Gibbs, D. L., Brown, S. D., Wolf, D., Bortone, D. S., Ou Yang, T. H. et al. The immune landscape of cancer. Immunity 48, 812–830 (2018).
Jamaspishvili, T., Berman, D. M., Ross, A. E., Scher, H. I., De Marzo, A. M., Squire, J. A. et al. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 15, 222–234 (2018).
Lee, J.-O., Yang, H., Georgescu, M.-M., Di Cristofano, A., Maehama, T., Shi, Y. et al. Crystal structure of the PTEN tumor suppressor. Cell 99, 323–334 (1999).
Steck, P. A., Pershouse, M. A., Jasser, S. A., Yung, W. K. A., Lin, H., Ligon, A. H. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 15, 356–362 (1997).
Maehama, T. & Dixon, J. E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 (1998).
Lee, J. O., Yang, H., Georgescu, M. M., Di Cristofano, A., Maehama, T., Shi, Y. et al. Crystal structure of the PTEN tumor suppressor: Implications for its phosphoinositide phosphatase activity and membrane association. Cell 99, 323–334 (1999).
Bassi, C., Ho, J., Srikumar, T., Dowling, R. J. O., Gorrini, C., Miller, S. J. et al. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science 341, 395–399 (2013).
Shen, W. H., Balajee, A. S., Wang, J., Wu, H., Eng, C., Pandolfi, P. P. et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128, 157–170 (2007).
Ma, J., Benitez, J. A., Li, J., Miki, S., Ponte de Albuquerque, C., Galatro, T. et al. Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell 35, 504–518 (2019).
Zhao, D., Lu, X., Wang, G., Lan, Z., Liao, W., Li, J. et al. Synthetic essentiality of chromatin remodelling factor CHD1 in PTEN-deficient cancer. Nature 542, 484–488 (2017).
Collado, M., Blasco, M. A. & Serrano, M. Cellular senescence in cancer and aging. Cell 130, 223–233 (2007).
Jung, S. H., Hwang, H. J., Kang, D., Park, H. A., Lee, H. C., Jeong, D. et al. mTOR kinase leads to PTEN-loss-induced cellular senescence by phosphorylating p53. Oncogene 38, 1639–1650 (2019).
Chen, Z., Trotman, L. C., Shaffer, D., Lin, H.-K., Dotan, Z. A., Niki, M. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005).
Song, M. S., Carracedo, A., Salmena, L., Song, S. J., Egia, A., Malumbres, M. et al. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell 144, 187–199 (2011).
Toso, A., Revandkar, A., DiMitri, D., Guccini, I., Proietti, M., Sarti, M. et al. Enhancing chemotherapy efficacy in pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 9, 75–89 (2014).
Vidotto, T., Tiezzi, D. G. & Squire, J. A. Distinct subtypes of genomic PTEN deletion size influence the landscape of aneuploidy and outcome in prostate cancer. Mol. Cytogenet. 11, 1–12 (2018).
Williams, J. L., Greer, P. A. & Squire, J. A. Recurrent copy number alterations in prostate cancer: an in silico meta-analysis of publicly available genomic data. Cancer Genet. 207, 474–488 (2014).
Taylor, A. M., Shih, J., Ha, G., Gao, G. F., Zhang, X., Berger, A. C. et al. Genomic and functional approaches to understanding cancer aneuploidy. Cancer Cell 33, 676–689 (2018).
Davoli, T., Uno, H., Wooten, E. C. & Elledge, S. J. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 355, 1–14 (2017).
Turajlic, S., Litchfield, K., Xu, H., Rosenthal, R., McGranahan, N., Reading, J. L. et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 18, 1009–1021 (2017).
Dudley, J. C., Lin, M.-T., Le, D. T. & Eshleman, J. R. Microsatellite instability as a biomarker for PD-1 blockade. Clin. Cancer Res. 22, 813–820 (2016).
Mittendorf, E. A., Philips, A. V., Meric-Bernstam, F., Qiao, N., Wu, Y., Harrington, S. et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2, 361–370 (2014).
Parsa, A. T., Waldron, J. S., Panner, A., Crane, C. A., Parney, I. F., Barry, J. J. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 13, 84–88 (2007).
Lastwika, K. J., Wilson, W., Li, Q. K., Norris, J., Xu, H., Ghazarian, S. R. et al. Control of PD-L1 expression by oncogenic activation of the AKT–mTOR pathway in non–small cell lung cancer. Cancer Res 76, 227–238 (2016).
Berghoff, A. S., Kiesel, B., Widhalm, G., Rajky, O., Ricken, G., Wohrer, A. et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 17, 1064–1075 (2015).
Garcia, A. J., Ruscetti, M., Arenzana, T. L., Tran, L. M., Bianci-Frias, D., Sybert, E. et al. Pten null prostate epithelium promotes localized myeloid-derived suppressor cell expansion and immune suppression during tumor initiation and progression. Mol. Cell Biol. 34, 2017–2028 (2014).
Miething, C., Scuoppo, C., Bosbach, B., Appelmann, I., Nakitandwe, J., Ma, J. et al. PTEN action in leukaemia dictated by the tissue microenvironment. Nature 510, 402–406 (2014).
Colombo, M. P. & Piconese, S. Regulatory T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat. Rev. Cancer 7, 880–887 (2007).
Ivashkiv, L. B. & Donlin, L. T. Regulation of type I interferon responses. Nat. Rev. Immunol. 14, 36–49 (2013).
Shen, H. & Lentsch, A. B. Progressive dysregulation of transcription factors NF-κB and STAT1 in prostate cancer cells causes proangiogenic production of CXC chemokines. Am. J. Physiol. Physiol. 286, 840–847 (2004).
Fuertes, M. B., Woo, S. R., Burnett, B., Fu, Y. X. & Gajewski, T. F. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 34, 67–73 (2013).
Parker, B. S., Rautela, J. & Hertzog, P. J. Antitumour actions of interferons: implications for cancer therapy. Nat. Rev. Cancer 16, 131–144 (2016).
Bakhoum, S. F. & Cantley, L. C. The multifaceted role of chromosomal instability in cancer and its microenvironment. Cell 174, 1347–1360 (2018).
Mouw, K. W., Goldberg, M. S., Konstantinopoulos, P. A. & D’Andrea, A. D. DNA damage and repair biomarkers of immunotherapy response. Cancer Discov. 7, 675–693 (2017).
Wang, H., Yu, Q., Wang, L., Li, Y., Xie, M., Lu, Y. et al. Expression of PTEN‑long nephritis and its effect on renal inflammation. Exp. Ther. Med. 17, 1405–1411 (2018).
Bazzichetto, C., Conciatori, F., Pallocca, M., Falcone, I., Fanciulli, M., Cognetti, F. et al. PTEN as a prognostic/predictive biomarker in cancer: an unfulfilled promise? Cancers (Basel) 11, 435–447 (2019).
Newton, R. H. & Turka, L. A. Regulation of T cell homeostasis and responses by Pten. Front. Immunol. 3, 1–9 (2012).
Shrestha, S., Yang, K., Guy, C., Vogel, P., Neale, G. & Chi, H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol. 16, 178–187 (2015).
Suzuki, A., Kaisho, T., Ohishi, M., Tsukio-Yamaguchi, M., Tsubata, T., Koni, P. A. et al. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J. Exp. Med. 197, 657–667 (2003).
Sahin, E., Haubenwallner, S., Kuttke, M., Kollmann, I., Halfmann, A., Dohnal, A. B. et al. Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J. Immunol. 193, 1717–1727 (2014).
Di Cristofano, A., Kotsi, P., Peng, Y. F., Cordon-Cardo, C., Elkon, K. B. & Pandolfi, P. P. Impaired Fas response and autoimmunity in Pten+/- mice. Science 285, 2122–2125 (1999).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Armstrong, C. W. D., Maxwell, P. J., Ong, C. W., Redmond, K. M., Mccann, C., Neisen, J. et al. PTEN deficiency promotes macrophage infiltration and hypersensitivity of prostate cancer to IAP antagonist/radiation combination therapy. Oncotarget 7, 7885–7898 (2016).
Chelouche-Lev, D., Miller, C. P., Tellez, C., Ruiz, M., Bar-Eli, M. & Price, J. E. Different signalling pathways regulate VEGF and IL-8 expression in breast cancer: implications for therapy. Eur. J. Cancer 40, 2509–2518 (2004).
Ying, H., Elpek, K. G., Vinjamoori, A., Zimmerman, S. M., Chu, G. C., Yan, H. et al. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-κB-cytokine network. Cancer Discov. 1, 158–169 (2011).
Loveridge, C. J., Mui, E. J., Patel, R., Tan, E. H., Ahmad, I., Welsh, M. et al. Increased T-cell infiltration elicited by Erk5 deletion in a Pten-deficient mouse model of prostate carcinogenesis. Cancer Res. 77, 3158–3168 (2017).
Phillips, R. J., Mestas, J., Gharaee-Kermani, M., Burdick, M. D., Sica, A., Belperio, J. A. et al. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1α. J. Biol. Chem. 280, 22473–22481 (2005).
Trimboli, A. J., Cantemir-Stone, C. Z., Li, F., Wallace, J. A., Merchant, A., Creasap, N. et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 461, 1084–1091 (2009).
Conley-LaComb, M., Saliganan, A., Kandagatla, P., Chen, Y. Q., Cher, M. L. & Chinni, S. R. PTEN loss mediated Akt activation promotes prostate tumor growth and metastasis via CXCL12/CXCR4 signaling. Mol. Cancer 12, 85–97 (2013).
Maxwell, P. J., Neisen, J., Messenger, J. & Waugh, D. J. J. Tumor-derived CXCL8 signaling augments stroma-derived CCL2-promoted proliferation and CXCL12-mediated invasion of PTEN-deficient prostate cancer cells. Oncotarget 5, 4895–4908 (2014).
Bezzi, M., Seitzer, N., Ishikawa, T., Reschke, M., Chen, M., Wang, G. et al. Diverse genetic-driven immune landscapes dictate tumor progression through distinct mechanisms. Nat. Med. 24, 165–175 (2018).
Hollander, M. C., Blumenthal, G. M. & Dennis, P. A. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer 11, 289–301 (2011).
Nieuwenhuis, M. H., Kets, C. M., Murphy-Ryan, M., Yntema, H. G., Evans, D. G., Colas, C. et al. Cancer risk and genotype-phenotype correlations in PTEN hamartoma tumor syndrome. Fam. Cancer 13, 57–63 (2014).
Sloot, Y. J. E., Rabold, K., Netea, M. G., Smit, J. W. A., Hoogerbrugge, N. & Netea-Maier, R. T. Effect of PTEN inactivating germline mutations on innate immune cell function and thyroid cancer-induced macrophages in patients with PTEN hamartoma tumor syndrome. Oncogene 38, 3743–3755 (2019).
Conciatori, F., Bazzichetto, C., Falcone, I., Pilotto, S., Bria, E., Cognetti, F. et al. Role of mTOR signaling in tumor microenvironment: an overview. Int. J. Mol. Sci. 19, 2453–2467 (2018).
Kaur, H. B., Guedes, L. B., Lu, J., Maldonado, L., Reitz, L., Barber, J. R. et al. Association of tumor-infiltrating T-cell density with molecular subtype, racial ancestry and clinical outcomes in prostate cancer. Mod. Pathol. 31, 1539–1552 (2018).
Bian, Y., Hall, B., Sun, Z.-J., Molinolo, A., Chen, W., Gutkind, J. S. et al. Loss of TGF-β signaling and PTEN promotes head and neck squamous cell carcinoma through cellular senescence evasion and cancer-related inflammation. Oncogene 31, 3322–3332 (2012).
Shabaneh, T. B., Molodtsov, A. K., Steinberg, S. M., Zhang, P., Torres, G. M., Mohamed, G. A. et al. Oncogenic BRAFV600E governs regulatory T-cell recruitment during melanoma tumorigenesis. Cancer Res 78, 5038–5049 (2018).
Peng, W., Chen, J. Q., Liu, C., Malu, S., Creasy, C., Tetzlaff, M. T. et al. Loss of PTEN promotes resistance to T cell–mediated immunotherapy. Cancer Discov. 6, 202–216 (2016).
Fridman, W. H., Zitvogel, L., Sautès-Fridman, C., Kroemer, G., Sautès–Fridman, C., Kroemer, G. et al. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 14, 717–734 (2017).
Gentles, A. J., Newman, A. M., Liu, C. L., Bratman, S. V., Feng, W., Kim, D. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015).
Jiménez-Sánchez, A., Memon, D., Pourpe, S., Veeraraghavan, H., Li, Y., Vargas, H. A. et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell 170, 927–938 (2017).
Liang, X., Daikoku, T., Terakawa, J., Ogawa, Y., Joshi, A. R., Ellenson, L. H. et al. The uterine epithelial loss of Pten is inefficient to induce endometrial cancer with intact stromal Pten. PLoS Genet. 14, 1–19 (2018).
Iwanaga, K., Yang, Y., Raso, M. G., Ma, L., Hanna, A. E., Thilaganathan, N. et al. Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer. Cancer Res. 68, 1119–1127 (2008).
Homet Moreno, B., Zaretsky, J. M., Garcia-Diaz, A., Tsoi, J., Parisi, G., Robert, L. et al. Response to programmed cell death-1 blockade in a murine melanoma syngeneic model requires costimulation, CD4, and CD8 T cells. Cancer Immunol. Res. 4, 845–857 (2016).
Almatroodi, S. A., McDonald, C. F., Darby, I. A. & Pouniotis, D. S. Characterization of M1/M2 tumour-associated macrophages (TAMs) and Th1/Th2 cytokine profiles in patients with NSCLC. Cancer Microenviron. 9, 1–11 (2016).
Chen, P., Zhao, D., Li, J., Liang, X., Li, J., Chang, A. et al. Symbiotic macrophage-glioma cell interactions reveal synthetic lethality in PTEN-null glioma. Cancer Cell 35, 868–884 (2019).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Di Mitri, D., Toso, A., Chen, J. J., Sarti, M., Pinton, S., Jost, T. R. et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 515, 134–137 (2014).
Limagne, E., Euvrard, R., Thibaudin, M., Rébé, C., Derangère, V., Chevriaux, A. et al. Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX–bevacizumab drug treatment regimen. Cancer Res. 76, 5241–5252 (2016).
Calcinotto, A., Spataro, C., Zagato, E., Di Mitri, D., Gil, V., Crespo, M. et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 559, 363–369 (2018).
Koyasu, S. The role of PI3K in immune cells. Nat. Immunol. 4, 313–319 (2003).
Okkenhaug, K., Graupera, M. & Vanhaesebroeck, B. Targeting PI3K in cancer: impact on tumor cells, their protective stroma, angiogenesis, and immunotherapy. Cancer Discov. 6, 1090–1105 (2016).
Pitt, J. M., Vétizou, M., Daillère, R., Roberti, M. P., Yamazaki, T., Routy, B. et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 44, 1255–1269 (2016).
Zhang, X., Zeng, Y., Qu, Q., Zhu, J., Liu, Z., Ning, W. et al. PD-L1 induced by IFN-γ from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer. Int. J. Clin. Oncol. 22, 1026–1033 (2017).
George, S., Miao, D., Demetri, G. D., Adeegbe, D., Rodig, S. J., Shukla, S. et al. Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity 46, 197–204 (2017).
Jin, H., Su, J., Garmy-Susini, B., Kleeman, J. & Varner, J. Integrin α4β1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res. 66, 2146–2152 (2006).
Roland, C. L., Dineen, S. P., Lynn, K. D., Sullivan, L. A., Dellinger, M. T., Sadegh, L. et al. Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Mol. Cancer Ther. 8, 1761–1771 (2009).
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15, 325–340 (2018).
Lastwika, K. J., Wilson, W., Li, Q. K., Norris, J., Xu, H., Ghazarian, S. R. et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 76, 227–238 (2016).
Dong, Y., Richards, J. A., Gupta, R., Aung, P. P., Emley, A., Kluger, Y. et al. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene 33, 4632–4642 (2014).
Feng, S., Cheng, X., Zhang, L., Lu, X., Chaudhary, S., Teng, R. et al. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc. Natl Acad. Sci. USA 115, 10094–10099 (2018).
Martin, A. M., Nirschl, T. R., Nirschl, C. J., Francica, B. J., Kochel, C. M., van Bokhoven, A. et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 18, 325–332 (2015).
de Bono, J. S., De Giorgi, U., Rodrigues, D. N., Massard, C., Bracarda, S., Font, A. et al. Randomized phase II study evaluating Akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin. Cancer Res. 25, 928–936 (2019).
Waldron, J. S., Yang, I., Han, S., Tihan, T., Sughrue, M. E., Mills, S. A. et al. Implications for immunotherapy of tumor-mediated T-cell apoptosis associated with loss of the tumor suppressor PTEN in glioblastoma. J. Clin. Neurosci. 17, 1543–1547 (2010).
Lu, B. & Finn, O. J. T-cell death and cancer immune tolerance. Cell Death Differ. 15, 70–79 (2008).
Marshall, N. A., Galvin, K. C., Corcoran, A.-M. B., Boon, L., Higgs, R. & Mills, K. H. G. Immunotherapy with PI3K inhibitor and toll-like receptor agonist induces IFN- +IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer Res. 72, 581–591 (2012).
McLoughlin, N. M., Mueller, C. & Grossmann, T. N. The therapeutic potential of PTEN modulation: targeting strategies from gene to protein. Cell Chem. Biol. 25, 19–29 (2018).
Lotan, T. L., Wei, W., Ludkovski, O., Morais, C. L., Guedes, L. B., Jamaspishvili, T. et al. Analytic validation of a clinical-grade PTEN immunohistochemistry assay in prostate cancer by comparison with PTEN FISH. Mod. Pathol. 29, 904–914 (2016).
Song, M., Chen, D., Lu, B., Wang, C., Zhang, J., Huang, L. et al. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS ONE 8, e65821 (2013).
Yang, R., Cai, T., Wu, X., Liu, Y., He, J., Zhang, X. et al. Tumour YAP1 and PTEN expression correlates with tumour-associated myeloid suppressor cell expansion and reduced survival in colorectal cancer. Immunology 155, 263–272 (2018).
Crumley, S., Kurnit, K., Hudgens, C., Fellman, B., Tetzlaff, M. T. & Broaddus, R. Identification of a subset of microsatellite-stable endometrial carcinoma with high PD-L1 and CD8+ lymphocytes. Mod. Pathol. 32, 396–404 (2019).
Li, N., Qin, J., Lan, L., Zhang, H., Liu, F., Wu, Z. et al. PTEN inhibits macrophage polarization from M1 to M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer Biol. Ther. 16, 297–306 (2015).
Kim, H. S., Lee, J. H., Nam, S. J., Ock, C. Y., Moon, J. W., Yoo, C. W. et al. Association of PD-L1 expression with tumor-infiltrating immune cells and mutation burden in high-grade neuroendocrine carcinoma of the lung. J. Thorac. Oncol. 13, 636–648 (2018).
Xu, C., Fillmore, C. M., Koyama, S., Wu, H., Zhao, Y., Chen, Z. et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD–L1 expression. Cancer Cell 25, 590–604 (2014).
Hlaing, A. M., Furusato, B., Udo, E., Kitamura, Y., Souda, M., Masutani, M. et al. Expression of phosphatase and tensin homolog and programmed cell death ligand 1 in adenosquamous carcinoma of the lung. Biochem. Biophys. Res. Commun. 503, 2764–2769 (2018).
Vidotto, T., Saggioro, F. P., Jamaspishvili, T., Chesca, D. L., Picanço de Albuquerque, C. G., Reis, R. B. et al. PTEN–deficient prostate cancer is associated with an immunosuppressive tumor microenvironment mediated by increased expression of IDO1 and infiltrating FoxP3+ T regulatory cells. Prostate 79, 969–979 (2019).
Jolly, L. A., Massoll, N. & Franco, A. T. Immune suppression mediated by myeloid and lymphoid derived immune cells in the tumor microenvironment facilitates progression of thyroid cancers driven by HrasG12V and Pten loss. J. Clin. Cell Immunol. 7, 451 (2016).
Acknowledgements
The authors thank Dr. Tamara Lotan for her helpful suggestions to improve this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this review.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Not applicable.
Competing interests
The authors declare no competing interests.
Funding information
This work was supported by São Paulo Research Foundation (awards #2015/22785-5, #2017/08614-9, and #2015/09111-5) and CNPq (award 306864/2014-2).
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vidotto, T., Melo, C.M., Castelli, E. et al. Emerging role of PTEN loss in evasion of the immune response to tumours. Br J Cancer 122, 1732–1743 (2020). https://doi.org/10.1038/s41416-020-0834-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-0834-6
This article is cited by
-
PTEN pathogenic variants are associated with poor prognosis in patients with advanced soft tissue sarcoma
BJC Reports (2024)
-
Mechanisms driving the immunoregulatory function of cancer cells
Nature Reviews Cancer (2023)
-
Pan-cancer genomic analysis shows hemizygous PTEN loss tumors are associated with immune evasion and poor outcome
Scientific Reports (2023)
-
Integrating somatic CNV and gene expression in breast cancers from women with PTEN hamartoma tumor syndrome
npj Genomic Medicine (2023)
-
Immunogenomic profiles associated with response to life-prolonging agents in prostate cancer
British Journal of Cancer (2023)