Abstract
Chemotherapy-related fatigue (CRF) is increasingly being recognized as one of the severe symptoms in patients undergoing chemotherapy, which not only largely reduces the quality of life in patients, but also diminishes their physical and social function. At present, there is no effective drug for preventing and treating CRF. Ganoderic acid (GA), isolated from traditional Chinese medicine Ganoderma lucidum, has shown a variety of pharmacological activities such as anti-tumor, anti-inflammation, immunoregulation, etc. In this study, we investigated whether GA possessed anti-fatigue activity against CRF. CT26 tumor-bearing mice were treated with 5-fluorouracil (5-FU, 30 mg/kg) and GA (50 mg/kg) alone or in combination for 18 days. Peripheral and central fatigue-related behaviors, energy metabolism and inflammatory factors were assessed. We demonstrated that co-administration of GA ameliorated 5-FU-induced peripheral muscle fatigue-like behavior via improving muscle quality and mitochondria function, increasing glycogen content and ATP production, reducing lactic acid content and LDH activity, and inhibiting p-AMPK, IL-6 and TNF-α expression in skeletal muscle. Co-administration of GA also retarded the 5-FU-induced central fatigue-like behavior accompanied by down-regulating the expression of IL-6, iNOS and COX2 in the hippocampus through inhibiting TLR4/Myd88/NF-κB pathway. These results suggest that GA could attenuate 5-FU-induced peripheral and central fatigue in tumor-bearing mice, which provides evidence for GA as a potential drug for treatment of CRF in clinic.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the fourth most common cancer in the world; there are more than 1.2 million patients with CRC, and 600,000 individuals die from the disease every year [1, 2]. 5-FU, one of the most widely used first-line chemotherapy agents for the treatment of CRC [3, 4], frequently causes fatigue and loss of appetite [5,6,7,8]. Chemotherapy-related fatigue (CRF) is increasingly recognized as a severe symptom in patients undergoing chemotherapy, and it can persist for years after treatments [9,10,11,12]; this condition not only largely reduces the quality of life of patients but also diminishes physical and social function, resulting in treatment limitations and increased morbidity [13].
CRF can result from both muscle dysfunction, termed ‘peripheral fatigue’, and decreased capacity of the central nervous system (CNS), termed ‘central fatigue’ [14]. Some studies have reported that chemotherapy leads to muscle fatigue by decreasing lean body and muscle mass [15, 16]. In addition, chemotherapy damages the skeletal musculature, inducing mitochondrial damage [17, 18], and leads to energy metabolism alterations and distinct metabolic derangements, including decreased energy supply and ATP production and increased lactate content [19]. Moreover, there is increasing evidence that proinflammatory cytokines, especially IL-6, TNF-α and IL-1β, in muscle and the nervous system significantly contribute to the occurrence of CRF [20,21,22,23,24], indicating that both muscle and central disorders are likely to play an essential role in CRF.
At present, there is no FDA-approved drug that can effectively prevent and treat CRF. Recently, increasing evidence has revealed that natural products derived from a variety of sources may be novel and promising alternative drugs for preventing and treating CRF [25,26,27] owing to their multiple pharmacological effects. Ganoderma lucidum (G. lucidum), a well-known traditional Chinese medicine [28], has various therapeutic properties, such as the ability to improve immunity, hepatic and renal function, as well as increase energy production, promote health and protect against the aging [29,30,31,32]. Clinical use of G. lucidum has revealed that it exerts beneficial effects in the context of CRF and on the overall quality of life in patients with cancer undergoing endocrine treatment by decreasing the concentrations of IL-6 and TNF-α [33]. Bioactive compounds that can be isolated from the mycelia and fruiting bodies of G. lucidum include triterpenes, polysaccharides, and other constituents, such as alkaloids, flavonoids, proteins, amino acids, mannitol, and steroids [34]. It has been reported that ganoderic acid (GA), the major component of Ganoderma triterpenes, reduces LPS-induced expression of IL-1β, IL-6 and TNF-α and alters mitochondrial metabolic activity [35] and neuronal inflammation [36], which may contribute to occurrence of CRF [20].
In this study, we investigated the effects of GA on 5-FU-induced peripheral muscle fatigue and central fatigue in colon tumor-bearing mice by performing a series of behavioral tests and evaluating biological parameters. Our experimental results showed that GA alleviated CRF by improving muscle quality, increasing energy metabolism and suppressing peripheral and neuronal inflammation, which indicates that GA may be developed as a novel drug for CRF treatment.
Materials and methods
Animals
Female BALB/c mice (6–7 weeks, 18–22 g body weight) were purchased from the Animal Center of Peking University Health Science Center (Beijing, China). Female mice were used in this study because tumor-bearing female mice maintain food intake and body weight better than male mice [37] and male mice often bite the tumor site [38]. The experiments were performed according to the National Institutes of Health Guidelines on the Use of Laboratory Animals. The University Animal Care Committee for Animal Research of Peking University Health Science Center approved the study protocol (Beijing, China). The mice were maintained in cages at 25 ± 1 °C on a 12 h light/dark cycle and provided free access to water and food throughout the experimental period. The animals were randomly divided into experimental groups (n = 10/group).
Drugs
GA was extracted and purified from the dried fruiting bodies of G. lucidum as reported previously [39, 40]. The concentrations of GA-A, GA-B, and GA-C2 were 16.101, 10.586, and 5.404 µg/mL, respectively. These three monomers accounted for 16.1% (GA-A), 10.6% (GA-B), and 5.4% (GA-C2) of crude GA. The purity of these three monomers was >98%, as determined by HPLC [39, 40]. GA was dissolved in saline with 5% Tween 80 and injected intraperitoneally at a dose of 50 mg/kg.
5-FU, which was purchased from Sigma (MO, USA), was dissolved in saline with 5% Tween 80 and injected intraperitoneally at a dose of 30 mg/kg according to previous research [41].
Tumor cell culture
The mouse colon adenocarcinoma cell line CT26 was provided by the Cell Resource Center, Institute of Basic Medicine, Chinese Academy of Medical Sciences (Beijing, China). The cells were cultured in RPMI-1640 medium (M&C, Beijing, China) with 10% fetal bovine serum (FBS; Gibco, Australia), 100 U/mL penicillin, and 100 μg/mL streptomycin in a humidified incubator with 5% CO2 at 37 °C. The cells were resuspended in PBS for injection.
Experimental tumor-bearing mouse model
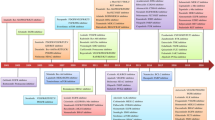
Mice were injected subcutaneously over the scapula with 200 μL of suspended tumor cells (2.5 × 105 cells/mouse) or PBS as described previously [42]. This site was selected as the tumor site instead of the right flank or an orthotopic site to avoid movement constraints that could interfere with the behavioral evaluations. It has been reported that this tumor model exhibits fatigue [37, 42, 43]. A treadmill adaptation test (TAT) was carried out before the tumors were palpable. Based on tumor size, the tumor-bearing mice were divided into four groups: the CT26 group, chemotherapy (5-FU, 30 mg/kg/2day) group, GA (GA, 50 mg/kg/day) group and chemotherapy combined with GA (5-FU + GA, 30 mg/kg/2day + 50 mg/kg/day) group. Tumor size was monitored twice a week using a digital caliper and calculated by the following formula: (width in mm)2 × (length in mm)/2. Body weight was recorded at the same time. As shown in Fig. 1a, the mice were subjected to behavioral tests on the drug intervention days.
GA (50 mg/kg), 5-FU (30 mg/kg) or normal saline with 5% Tween 80 was intraperitoneally administered to female BALB/c tumor-bearing mice for 18 days after grouping. a Study design. b Body weight. c Tumor free body weight. d Tumor volume. e Tumor to body weight. The values are shown as the mean ± SEM (n = 10). **P < 0.01, ***P < 0.001 vs the control group. #P < 0.05, ##P < 0.01, ###P < 0.001 vs the CT26 group. $P < 0.05 vs the CT26 + 5-FU group. TAT treadmill adaptation test, GST grip strength test, TFT treadmill fatigue test, OFT open field test, TST tail suspension test, CON control, CT26 CT26 tumor-bearing mice, 5-FU 5-fluorouracil, GA ganoderic acid, 5-FU + GA 5-fluorouracil combined with ganoderic acid.
Grip strength test (GST)
The muscle strength of the mice was assessed weekly using a commercial digital grip strength meter with an attached metal grid (YLS-13A, Shanghai, China). The mice were lifted and held by the tail so that the forepaws could grasp the wire grid, which was connected to a dynamometer. The mice were then gently pulled backward by the tail until they released their grip. The peak force of the forelimbs was recorded. Three trials were performed for each mouse, and the results were used for statistical analysis.
Treadmill fatigue test (TFT)
The TFT was performed according to a previously described method [42] with minor modifications. In brief, a mouse treadmill (Zhongshidichuang, Beijing, China) with 10-degree incline and a grid that delivered 1.2 mA electric shocks at 2 Hz was used for training and testing. The mice were acclimated for 3 consecutive days before the tumors were palpable. The fatigue test was conducted on day 8 and day 15, and the following program was used: 12 m/min for 5 min, 14 m/min for 5 min, 17 m/min for 3 min, 20 m/min for 3 min, 23 m/min for 3 min, 26 m/min for 5 min, and 29 m/min until the end of the test. The experimenter did not interact with the animals until they met the criterion for fatigue-like behavior, which was defined as remaining in the designated fatigue zone of the treadmill on the electric grid for 5 s. Fatigue-like behavior in each run-to-exhaustion trial was estimated using the running distance.
Open field test (OFT)
Central fatigue-like behavior was measured by using the open field test (OFT) as reported previously [44]. Mice were individually placed in the center of the open field apparatus (50 cm × 50 cm × 50 cm) and observed for 5 min. Locomotor activity, such as distance traveled, rest time, speed while mobile, ratio of time spent in the central zone and the number of entries into the central zone was assessed with an animal tracking system (Zhongshidichuang, Beijing, China).
Tail suspension test (TST)
It has been reported that the tail suspension test (TST), which is typically used to evaluate depression-like behavior, can measure fatigue-like behavior in mice [45,46,47], and recently, researchers have used the TST to assess mental fatigue-like behavior related to cancer-related fatigue [48]. The TST was performed according to the method reported by Streru [37]. The mice were hung 50 cm above the floor with adhesive tape, which was placed ~1 cm from the top of the tail. The total time of immobility over 6 min was recorded.
Biochemical assessments
After the behavioral tests, the mice were deeply euthanized by inhaled isoflurane. Serum was collected by centrifuging whole blood at 3500 r/min for 15 min. The gastrocnemius muscles, tibialis anterior muscles, quadriceps muscles and tumors were excised and weighed, frozen in liquid nitrogen, and kept at −80 °C for further studies. Serum samples were subjected to biochemical assays, including assays of lactate dehydrogenase (LDH) and lactic acid (LD) (NJJC Bio, Nanjing, Jiangsu, China), ATP (Vigorous Biotechnology, Beijing, China), IL-6, IL-1β and IL-15 (Applygen, Beijing, China) levels with biochemical kits. Glycogen in the liver and muscle was hydrolyzed in aqueous alkali solution, and the glycogen content was determined with a biochemical kit (NJJC Bio, Nanjing, Jiangsu, China). All assays were carried out according to the manufacturers’ instructions.
Histological staining and ultrastructural examination
Skeletal muscles were carefully collected, minced, fixed in 10% formalin in 0.01 M phosphate-buffered saline (PBS), dehydrated, embedded in paraffin, and cut into 5 μm-thick slices. After deparaffinization, the skeletal muscle sections were rehydrated, stained with hematoxylin and eosin, and examined under a microscope for morphological and pathological evaluation.
For ultrastructural evaluation, skeletal muscles that were cut into 1 mm3 pieces were fixed in 2.5% glutaraldehyde, postfixed in osmium tetroxide, and stained with uranyl acetate and lead citrate. The samples were thinly sectioned and observed under a transmission electron microscope, and ultrastructural changes in mitochondria were examined.
Western blot analysis
Protein was extracted from the skeletal muscles and hippocampi of mice from different groups by homogenization using tissue protein lysis buffer (Mei5, MF188-01, Beijing, China) supplemented with protease inhibitor cocktail (Roche, Basel, Kanton Basel-Stadt, Switzerland). The concentrations of the protein samples were determined by using a Bicinchoninic Acid Protein Assay Reagent Kit (Pierce, Rockford, IL, USA). Equal amounts of proteins were loaded on gels, separated by SDS-PAGE, and then transferred to polyvinylidene difluoride membranes (Amersham Biosciences, Boston, MA, USA). After blocking, the membranes were incubated overnight at 4 °C with homologous primary antibodies against β-actin (1:10000, ABclonal, AC026, Wuhan, China), p-AMPK (1:2500, CST, 2535, MA, USA), AMPK (1:2500, CST, 2523, MA, USA), IL-6 (1:1000, ABclonal, A0286, Wuhan, China), IL-1β (1:1000, ABclonal, A11370, Wuhan, China), TNF-α (1:1000, ABclonal, A0277, Wuhan, China), COX2 (1:1000, ABclonal, A1253, Wuhan, China), iNOS (1:1000, ABclonal, A0312, Wuhan, China), Myd88 (1:1000, ABclonal, A0980, Wuhan, China), TLR4 (1:1000, ABclonal, A11226, Wuhan, China), and NF-κB (1:1000, Immunoway, YM3111, TX, USA), and the blots were developed with an ECL plus kit (Biodragon, Beijing, China). The protein bands were visualized with a chemiluminescence detection system (Syngene, GeneGnome XRQ, Cambridge, Cambridgeshire, UK) and analyzed by ImageJ software (NIH, MD, USA).
Statistical analysis
The statistical data were plotted with GraphPad Prism software (GraphPad Software, Inc., San Diego, CA, USA). For multiple comparisons, statistical analysis was performed using Student’s t test or one-way ANOVA or two-way ANOVA. P < 0.05 was considered statistically significant. All data are expressed as the mean ± SEM.
Results
GA significantly inhibited colon tumor growth in CT26 tumor-bearing mice
As shown in Fig. 1b, compared to control group, body weight of CT26 tumor-bearing mice was significantly increased from day 12 to day 16, which may be due to the increased tumor burden, because the tumor free body weight of CT26 tumor-bearing mice did not differ from the mice in control group (Fig. 1c). Co-administration of GA increased the body weight and tumor-free body weight of chemotherapy-treated mice compared to the CT26 + 5-FU group (Fig. 1b, c). Furthermore, in mice that subcutaneously received CT26 tumor cells, the tumor burden rapidly increased from day 8 in the absence of drug intervention, while tumor growth was significantly inhibited (Fig. 1d) and tumor to body weight was reduced (Fig. 1e) following the treatment with GA and 5-FU. During the experiment, none of the mortality and macroscopic metastases were found upon gross visual examination of the livers, lungs, hearts, and kidneys of tumor-bearing mice treated with or without GA.
GA attenuated peripheral muscle fatigue-like behavior in chemotherapy-treated CT26 tumor-bearing mice
The grip strength test (GST) showed that muscle strength was identical among all groups on day 0 (Fig. 2a) but that muscle strength in the CT26 group and 5-FU-treated group were significantly decreased on day 7 (Fig. 2b) and more evidently decreased on day 14 compared to control group (Fig. 2c). Muscle strength was markedly improved in the GA-treated and GA plus 5-FU-treated groups compared to the CT26 group and 5-FU-treated group. Running distance on the treadmill was significantly decreased in the CT26 group and 5-FU-treated group compared to the control group on day 8 and day 15, while this change was significantly alleviated in the GA-treated and GA plus 5-FU-treated groups (Fig. 2d, e). Muscle mass, including gastrocnemius muscle (Fig. 2f), tibialis anterior muscle (Fig. 2g), quadriceps muscle (Fig. 2h) and total skeletal muscle mass (Fig. 2i), was reduced in CT26 tumor-bearing mice and 5-FU-treated mice compared to control mice. GA significantly rescued the depletion of these muscles’ mass in 5-FU-treated mice.
a Grip strength on day 0. b Grip strength on day 7. c Grip strength on day 14. d Running distance on day 8. e Running distance on day 15. f Gastrocnemius muscle weight to initial body weight (IBW) ratio. g Tibialis anterior muscle weight to IBW ratio. h Quadriceps muscle weight to IBW ratio. i Total skeletal muscle weight to IBW ratio. The values are shown as the mean ± SEM (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001 vs the control group. #P < 0.05 vs the CT26 group. $P < 0.05, $$P < 0.01, $$$P < 0.001 vs the CT26 + 5-FU group. CON control, CT26 CT26 tumor-bearing mice, 5-FU 5-fluorouracil, GA ganoderic acid, 5-FU + GA 5-fluorouracil combined with ganoderic acid.
GA alleviated skeletal muscle injury in chemotherapy-treated tumor-bearing mice
H&E staining (Fig. 3a) revealed a long column of skeletal muscle fibers with regular morphology, uniformly distributed nuclei under an intact myolemma, and clear periodic streaks of light and dark fibers in the control group and GA-treated group. There was no obvious change in muscle sections from CT26 mice, but 5-FU-treated tumor-bearing mice exhibited characteristic muscle pathology, such as disorganized skeletal muscle fibers, irregular cell morphology, broken muscle fibers, and inflammatory cell infiltration. Interestingly, GA reversed 5-FU-induced skeletal muscle pathological alterations in tumor-bearing mice, as shown in Fig. 3a.
a Representative micrographs of H&E staining (magnification of ×200; typical myofibrillar fragmentation is indicated by the yellow arrows). b Representative transmission electron microscopy (TEM) micrographs (magnification of ×15,000 or ×40,000, typical mitochondria, dissolving myofilaments and mitochondria swelling are indicated by mt, the yellow stars and the blue triangles, respectively). CT26 CT26 tumor-bearing mice, 5-FU 5-fluorouracil, GA ganoderic acid, 5-FU + GA 5-fluorouracil combined with ganoderic acid.
TEM assessment of the morphology of skeletal muscles (Fig. 3b) also showed that CT26 tumor-bearing mice and 5-FU-treated mice exhibited a series of alterations, such as disordered myofibril arrangement; shortened sarcomere length; dissolved or disappeared myofilaments; sarcoplasmic reticulum expansion; intracellular myelocystic bodies; and swollen, deformed, and even vacuole-like mitochondria. These mitochondrial changes were more pronounced in the 5-FU-treated mice than in the CT26 tumor-bearing mice, and GA effectively protected mitochondria from tumor- or 5-FU-induced dysfunction.
GA improved energy metabolism abnormalities and the levels of inflammatory cytokines in skeletal muscle in 5-FU-treated tumor-bearing mice
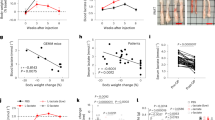
To evaluate the effect of GA on energy metabolism, we measured the serum ATP, LDH, LD, liver, and muscle glycogen contents and the relative protein expression levels of p-AMPK, AMPK, TNF-α, and IL-6. The results showed that while serum ATP content decreased (Fig. 4a), serum LDH activity and LD content increased in the CT26 group or the 5-FU-treated group (Fig. 4b, c). Fortunately, these alterations were significantly alleviated in the GA combined with 5-FU group. Muscle and liver glycogen contents were decreased in the 5-FU-treated group and CT26 group, but liver glycogen content was only significantly reduced in the 5-FU-treated group. Furthermore, GA rescued the decreases in muscle and liver glycogen contents in 5-FU-treated tumor-bearing mice (Fig. 4d, e). Furthermore, we measured the protein expression of p-AMPK, an energy stress sensor, to better understand energy balance and found that the relative expression of p-AMPK/AMPK was markedly upregulated in 5-FU-treated tumor-bearing mice. GA successfully downregulated the expression of p-AMPK/AMPK in chemotherapy-treated tumor-bearing mice (Fig. 4f, g).
a Serum ATP content (% of CON). b Serum LDH activity. c Serum LD content. d Liver glycogen content. e Muscle glycogen content. f Representative Western blots showing p-AMPK, AMPK, IL-6 and TNF-α protein expression in skeletal muscle. g Relative protein levels in the experiments shown in (f). The data were normalized to the intensity of β-actin or the nonphosphorylated form of the protein of interest and are expressed relative to the value of the control group. The values are shown as the mean ± SEM (n = 5–10). *P < 0.05, **P < 0.01, ***P < 0.001 vs the control group. $P < 0.05, $$P < 0.01 vs the CT26 + 5-FU group. CON control, CT26 CT26 tumor-bearing mice, 5-FU 5-fluorouracil, GA ganoderic acid, 5-FU + GA 5-fluorouracil combined with ganoderic acid, ATP adenosine-triphosphate, LDH lactate dehydrogenase, LD lactic acid, p-AMPK phosphorylated 5′-AMP-activated protein kinase, IL-6 interleukin-6, TNF-α tumor necrosis factor alpha.
To determine whether 5-FU could activate skeletal muscle inflammation in CT26 tumor-bearing mice and whether GA could modulate chemotherapy-related inflammatory activation in mice with CRF, we analyzed the expression of proinflammatory cytokines in skeletal muscle, including IL-6 and TNF-α. As shown in Fig. 4f, g, the expression of IL-6 and TNF-α in skeletal muscle was significantly upregulated in tumor-bearing mice treated with 5-FU, and fortunately, we found that GA significantly downregulated the expression of these proinflammatory cytokines in 5-FU-treated tumor-bearing mice.
The central fatigue-like behavior of chemotherapy-treated tumor-bearing mice was attenuated by GA
The results of the open field test (OFT) showed that the rest time (Fig. 5a) was obviously increased while speed while mobile (Fig. 5b), traveled distance (Fig. 5c), the time spent in the central zone (Supplementary Fig. S1a) and the number of central zone entries (Supplementary Fig. S1b) were significantly decreased in the CT26 group and 5-FU-treated group compared to the control group. GA reversed the abnormal changes in the rest time and traveled distance, but not increased the speed while mobile, time spent in the central zone or the number of central zone entries in the 5-FU-treated tumor-bearing mice. The immobility time (Fig. 5d) in the TST was increased in both CT26 tumor-bearing mice and 5-FU-treated tumor-bearing mice; furthermore, GA-treated mice showed no obvious change in immobility time, but the immobility time of the GA combined with 5-FU-treated mice was significantly lower than that of the 5-FU-treated mice.
a Rest time in the open field test (OFT). b Speed while mobile in the OFT. c Total distance traveled in the OFT. d Immobility time in the tail suspension test (TST). The values are shown as the mean ± SEM (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001 vs the control group. #P < 0.05 vs the CT26 group. $P < 0.05, $$P < 0.01 vs the CT26 + 5-FU group. CON control, CT26 CT26 tumor-bearing mice, 5-FU 5-fluorouracil, GA ganoderic acid, 5-FU + GA 5-fluorouracil combined with ganoderic acid.
GA hindered the expression of inflammatory cytokines and mediators in the hippocampi of colon tumor-bearing mice treated with 5-FU
We found that the serum contents of IL-6 (Supplementary Fig. S2a) and IL-1β (Supplementary Fig. S2b) were increased in 5-FU-treated tumor-bearing mice and that the serum content of IL-15 (Supplementary Fig. S2c) was markedly decreased in the CT26 group and 5-FU-treated group. GA suppressed the expression of IL-6 and IL-1β in the CT26- or 5-FU-treated group but did not increase the content of IL-15. Moreover, 5-FU treatment not only increased the levels of the proinflammatory cytokine IL-6 and the inflammatory response factors iNOS and COX2 (Fig. 6a, b), but also upregulated the expression of the innate immune response molecules TLR4, Myd88 and the transcription factor NF-κB in the hippocampus (Fig. 6c, d). Interestingly, GA effectively downregulated the expression of these over-activated inflammatory cytokines and mediators in the GA combined with 5-FU-treated group, suggesting that GA could relieve the central fatigue-like behavior of 5-FU-treated tumor-bearing mice by inhibiting neuroinflammation.
a Representative Western blots showing the expression of key proteins, namely, IL-6, IL-1β, TNF-α, COX2 and iNOS in the hippocampus. b Relative protein levels in the experiments shown in (a). The data were normalized to the intensity of β-actin and are expressed relative to the value of the control group. c Representative Western blots showing the protein expression of TLR4, Myd88 and NF-κB in the hippocampus. d Relative protein levels in the experiments shown in (c). The data were normalized to the intensity of β-actin and are expressed relative to the value of the control group. The values are shown as the mean ± SEM (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001 vs the control group. #P < 0.05 vs the CT26 group. $P < 0.05, $$P < 0.01, $$$P < 0.001 vs the CT26 + 5-FU group. CON control, CT26 CT26 tumor-bearing mice, 5-FU 5-fluorouracil, GA ganoderic acid, 5-FU + GA 5-fluorouracil combined with ganoderic acid, IL-6 interleukin-6, TNF-α tumor necrosis factor alpha, IL-1β interleukin-1beta, COX2 cyclooxygenase 2, iNOS inducible nitric oxide synthase, TLR4 Toll-like receptor 4, Myd88 myeloid differentiation factor 88, NF-κB nuclear factor kappa B.
Discussion
Fatigue is one of the most prevalent symptoms in cancer patients that complain of tiredness before, during, and after cancer treatment [9, 10, 49], and it decreases quality of life [13]. CRF prevents patients from making full use of adequate cytotoxic chemotherapy to improve life span. Previous studies have shown that GA, the major component of Ganoderma triterpenes, not only exhibits anti-tumor effects but also displays anti-inflammatory, anti-oxidative and immunomodulatory activities [50], which may affect the pathogenesis of CRF [20], suggesting that GA may have effects against CRF. In the present study, we found that GA effectively hindered fatigue-like behavior in 5-FU-treated mice, as evidenced by improvements in muscle mass and energy metabolism and modulation of peripheral muscle inflammation and neuroinflammation.
The etiology of CRF is complex, encompassing both peripheral and central mechanisms [20, 22, 23], which have been hypothesized to contribute to overall fatigue in cancer patients [51]. Generally, cancer-related fatigue is mainly induced by energy metabolism disruption in skeletal muscles [19, 23] and neuroinflammation in the CNS [22, 37, 52].
Peripheral fatigue refers to skeletal muscle fatigability or an inability to sustain force over time [14], most notably due to reductions in muscle strength and muscle mass, energy source and ATP depletion, and excess metabolite accumulation [53]. Some researchers have also reported that loss of muscle mass is common before [54] and after cancer treatment [19]. Recently, a clinical study also suggested that improving muscle mass is an effective strategy for ameliorating fatigue in patients with advanced cancer [16]. In this study, we found that GA could improve muscle mass, including the mass of the gastrocnemius, tibialis anterior and quadriceps muscles, and alleviate the decreases in grip strength and treadmill running in 5-FU-treated tumor-bearing mice, which may have been induced by reduced muscle mass.
Skeletal muscle is a highly metabolic organ that requires generation of a sufficient amount of ATP. Therefore, decreased ATP production eventually results in skeletal muscle dysfunction and fatigue [55]. In this study, we found a series of mitochondrial structural impairments, decreased muscle and liver glycogen content, decreased circulating ATP levels, and elevated LD content and LDH activity in 5-FU-treated tumor-bearing mice, indicating that balance of energy metabolism is clearly impacted by CRF. Furthermore, under glycolytic conditions, glycogen is rapidly consumed to produce a large amount of LD [56] and leads to muscle fatigue [57].
The energy-sensing molecule 5′-AMP-activated protein kinase (AMPK), as an indicator of cellular energy levels in response to metabolic stress [58], is activated under conditions of energy stress when intracellular ATP levels decline and intracellular AMP levels increase (high intracellular AMP/ATP ratio) [59]. Recently, researchers found that inflammation, such as elevated IL-6 and TNF-α levels, is linked to cachexia-induced mitochondrial dysfunction in skeletal muscle [60, 61]. It was also reported that an increase in IL-6 content in cachexia mice can lead to the activation of AMPK, whereas blockage of IL-6 ameliorates cachexia-induced AMPK activation [62], and reported that the activated AMPK causes decreased muscle mass [61], suggesting that chronic activation of AMPK by cytokines results in mitochondrial dysfunction, leading to lack of an appropriate energy supply for cell function, and loss of muscle mass and strength. Interestingly, GA significantly hindered 5-FU-induced mitochondrial dysfunction, depletion of energy sources and ATP generation, glycolytic metabolite accumulation, and upregulation of p-AMPK expression, which indicates that enhancing energy metabolism may be involved, at least in part, in the anti-peripheral muscle fatigue effects of GA.
Increasing evidence indicates that enhanced levels of inflammatory cytokines induced by chemotherapy may be closely associated with CRF [10, 63, 64]. Cytokines, such as IL-6, IL-1β and TNF-α, play important roles in the development of CRF [20, 21, 41]. These cytokines act as cell-to-cell mediators in response to external immunologic stressors, trigger immune-to-brain communication signaling in the CNS and alter behaviors [65]. Peripherally released cytokines are able to activate immune-to-brain communication pathways [66], enter the brain through various routes to activate astrocytes and microglia, and eventually trigger the generation of cytokines, inflammatory mediators and other neurotoxins, which induce neuroinflammation in the CNS [67].
Some researchers have demonstrated that neuroinflammation, especially that caused by proinflammatory cytokines in the hippocampus, contributes to decreased voluntary activity and fatigue [37, 47, 68]. Although TLR4 and the downstream molecule NF-κB are widely investigated as the mediators of inflammatory response and cytokine-mediated processes, limited studies have focused on the role of the TLR4 signaling pathway in chemotherapy-induced fatigue. It was reported that after stimulation of TLR4 by LPS, the Myd88-dependent pathway activates NF-κB, which induces the production of proinflammatory cytokines and mediators, including IL-6, iNOS and COX2 [69, 70]. Some researchers have also reported that COX2 inhibitors not only increase the effectiveness of chemoradiation [71] but also improve cancer patients’ quality of life via suppression of the proinflammatory cytokine pathway [72]. Others have found that Myd88, a key component of both the innate and adaptive immune systems and a universal adaptor protein for TLR4, plays a crucial role in mediating cancer-induced inflammation and that blocking this pathway alleviates pancreatic ductal adenocarcinoma-associated fatigue [73].
Surprisingly, we found that 5-FU led to significant overactivation of IL-6, iNOS and COX2 via the TLR4/Myd88/NF-κB-dependent pathway, whereas GA prevented the overactivation of the TLR4/Myd88/NF-κB signaling pathway in the hippocampus to alleviate central fatigue in chemotherapy-treated tumor-bearing mice. Abnormal activation of these cytokines was not found in the tumor-bearing mice, although there was an increasing trend in the expression of these cytokines and mediators in hippocampus; these changes are in line with previous research [74, 75]. Other researchers have also reported that chemotherapy triggers the production and secretion of inflammatory cytokines by tumor or immune cells [76]. Therefore, we hypothesized that inoculation of female BALB/c mice with CT26 cells in the subcutaneous space over the scapula might not be directly involved in cytokine abnormalities but that chemotherapy may contribute to the induction of inflammatory cytokines in tumor-bearing mice.
The limitations of this study must be acknowledged. To avoid possible behavior constraints due to tumor growth, we injected tumor cells into the subcutaneous space above the scapula instead of the right flank or an orthotopic site. Mice bearing subcutaneous tumors over the scapula present fatigue-like behavior with no movement restriction [37, 42, 72], but the microenvironment in this model may differ from that in models injected into the right flank or an orthotopic site. Therefore, it is imperative to study orthotopic mouse models, which better recapitulate the conditions of patients in the clinic.
In summary, as shown in Fig. 7, our study provides the first evidence that GA exerts a therapeutic effect against CRF in tumor-bearing mice. Based on our experiment, GA not only has good anti-tumor activity but also alleviates peripheral muscle fatigue and central fatigue-like behavior by improving muscle quality and emotional impairment. Moreover, GA can alleviate peripheral muscle fatigue by relieving decreased glycogen content, enhanced glycolysis, decreased ATP production and chronic activation of AMPK via inhibition of IL-6 and TNF-α in 5-FU-treated tumor-bearing mice. Finally, GA effectively inhibits TLR4-mediated Myd88 activation, which decreases the release of proinflammatory cytokines and mediators through the NF-κB pathway. Therefore, our study suggests that GA can be developed as a promising auxiliary therapeutic agent for CRF and is an alternative treatment for improving the quality of life of 5-FU-treated patients with colon cancer.
References
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80.
Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502.
Lee JJ, Beumer JH, Chu E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother Pharmacol. 2016;78:447–64.
McQuade RM, Stojanovska V, Bornstein JC, Nurgali K. Colorectal cancer chemotherapy: the evolution of treatment and new approaches. Curr Med Chem. 2017;24:1537–57.
Iacovelli R, Pietrantonio F, Maggi C, de Braud F, Di, Bartolomeo M. Combination or single-agent chemotherapy as adjuvant treatment of gastric cancer: A systematic review and meta-analysis of published trials. Crit Rev Oncol Hematol. 2016;98:24–8.
Dougherty JP, Springer DA, Cullen MJ, Gershengorn MC. Evaluation of the effects of chemotherapy-induced fatigue and pharmacological interventions in multiple mouse behavioral assays. Behav Brain Res. 2019;360:255–61.
Chakravarthy AB, Zhao F, Meropol NJ, Flynn PJ, Wagner LI, Sloan J, et al. Intergroup randomized phase III study of postoperative oxaliplatin, 5-fluorouracil, and leucovorin versus oxaliplatin, 5-fluorouracil, leucovorin, and bevacizumab for patients with stage II or III rectal cancer receiving preoperative chemoradiation: A trial of the ECOG-ACRIN research group (E5204). Oncologist. 2020;25:e798–e807.
Chibaudel B, Bachet JB, André T, Auby D, Desramé J, Deplanque G, et al. Efficacy of aflibercept with FOLFOX and maintenance with fluoropyrimidine as first‑line therapy for metastatic colorectal cancer: GERCOR VELVET phase II study. Int J Oncol. 2019;54:1433–45.
Husson O, Nieuwlaat WA, Oranje WA, Haak HR, van de Poll-Franse LV, Mols F. Fatigue among short- and long-term thyroid cancer survivors: results from the population-based PROFILES registry. Thyroid. 2013;23:1247–55.
Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30:S48–57.
Akin S, Kas Guner C. Investigation of the relationship among fatigue, self-efficacy and quality of life during chemotherapy in patients with breast, lung or gastrointestinal cancer. Eur J Cancer Care (Engl). 2019;28:e12898.
Xiao Z, Hu L, Lin J, Lu L, Huang X, Zhu X, et al. Efficacy and safety of Jianpishengsui for chemotherapy-related fatigue in patients with non-small cell lung cancer: study protocol for a randomized placebo-controlled clinical trial. Trials. 2020;21:94.
Vissers PA, Thong MS, Pouwer F, Zanders MM, Coebergh JW, van de Poll-Franse LV. The impact of comorbidity on health-related quality of life among cancer survivors: analyses of data from the PROFILES registry. J Cancer Surviv. 2013;7:602–13.
McMorris T, Barwood M, Corbett J. Central fatigue theory and endurance exercise: toward an interoceptive model. Neurosci Biobehav Rev. 2018;93:93–107.
Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–404.
Neefjes ECW, van den Hurk RM, Blauwhoff-Buskermolen S, van der Vorst M, Becker-Commissaris A, de van der Schueren MAE, et al. Muscle mass as a target to reduce fatigue in patients with advanced cancer. J Cachexia Sarcopenia Muscle. 2017;8:623–9.
Ballarò R, Beltrà M, De Lucia S, Pin F, Ranjbar K, Hulmi JJ, et al. Moderate exercise in mice improves cancer plus chemotherapy-induced muscle wasting and mitochondrial alterations. FASEB J. 2019;33:5482–94.
Barreto R, Mandili G, Witzmann FA, Novelli F, Zimmers TA, Bonetto A. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol. 2016;7:472.
Pin F, Barreto R, Couch ME, Bonetto A, O’Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle. 2019;10:140–54.
Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609.
Weymann KB, Wood LJ, Zhu X, Marks DL. A role for orexin in cytotoxic chemotherapy-induced fatigue. Brain Behav Immun. 2014;37:84–94.
Yang S, Chu S, Gao Y, Ai Q, Liu Y, Li X, et al. A narrative review of cancer-related fatigue (CRF) and its possible pathogenesis. Cells. 2019;8:738.
O’Higgins CM, Brady B, O’Connor B, Walsh D, Reilly RB. The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer. 2018;26:3353–64.
van Vulpen JK, Schmidt ME, Velthuis MJ, Wiskemann J, Schneeweiss A, Vermeulen RCH, et al. Effects of physical exercise on markers of inflammation in breast cancer patients during adjuvant chemotherapy. Breast Cancer Res. Treat. 2018;168:421–31.
Barton DL, Liu H, Dakhil SR, Linquist B, Sloan JA, Nichols CR, et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst. 2013;105:1230–8.
Inglis JE, Lin PJ, Kerns SL, Kleckner IR, Kleckner AS, Castillo DA, et al. Nutritional interventions for treating cancer-related fatigue: a qualitative review. Nutr Cancer. 2019;71:21–40.
Ouyang M, Liu Y, Tan W, Xiao Y, Yu K, Sun X, et al. Bu-zhong-yi-qi pill alleviate the chemotherapy-related fatigue in 4 T1 murine breast cancer model. BMC Complement Alter Med. 2014;14:497.
Hsu CL, Yen GC. Ganoderic acid and lucidenic acid (triterpenoid). Enzymes. 2014;36:33–56.
Wang J, Cao B, Zhao H, Feng J. Emerging roles of Ganoderma Lucidum in anti-aging. Aging Dis. 2017;8:691–707.
Su L, Liu L, Jia Y, Lei L, Liu J, Zhu S, et al. Ganoderma triterpenes retard renal cyst development by downregulating Ras/MAPK signaling and promoting cell differentiation. Kidney Int. 2017;92:1404–18.
Zhong D, Wang H, Liu M, Li X, Huang M, Zhou H, et al. Ganoderma lucidum polysaccharide peptide prevents renal ischemia reperfusion injury via counteracting oxidative stress. Sci Rep. 2015;5:16910.
Meng J, Wang SZ, He JZ, Zhu S, Huang BY, Wang SY, et al. Ganoderic acid A is the effective ingredient of ganoderma triterpenes in retarding renal cyst development in polycystic kidney disease. Acta Pharmacol Sin. 2020;41:782–90.
Zhao H, Zhang Q, Zhao L, Huang X, Wang J, Kang X. Spore powder of ganoderma lucidum improves cancer-related fatigue in breast cancer patients undergoing endocrine therapy: a pilot clinical trial. Evid Based Complement Altern Med. 2012;2012:809614.
Gill BS, Navgeet, Kumar S. Ganoderma lucidum targeting lung cancer signaling: a review. Tumour Biol. 2017;39:1010428317707437.
Chi B, Wang S, Bi S, Qin W, Wu D, Luo Z, et al. Effects of ganoderic acid A on lipopolysaccharide-induced proinflammatory cytokine release from primary mouse microglia cultures. Exp Ther Med. 2018;15:847–53.
Sheng F, Zhang L, Wang S, Yang L, Li P. Deacetyl ganoderic acid F inhibits LPS-induced neural inflammation via NF-κB pathway both in vitro and In vivo. Nutrients. 2019;12:85.
Norden DM, Bicer S, Clark Y, Jing R, Henry CJ, Wold LE, et al. Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function. Brain Behav Immun. 2015;43:76–85.
Yang M, Kim J, Kim JS, Kim SH, Kim JC, Kang MJ, et al. Hippocampal dysfunctions in tumor-bearing mice. Brain Behav Immun. 2014;36:147–55.
Lin DM, Wang SZ, Luo HJ, Lin ZX, Lin SQ. Rapid separation of ganoderic acid from the extraction by-products of Ganoderma lucidum. Fujian Med J. 2018;40:135–8.
Geng XQ, Ma A, He JZ, Wang L, Jia YL, Shao GY, et al. Ganoderic acid hinders renal fibrosis via suppressing the TGF-β/Smad and MAPK signaling pathways. Acta Pharmacol Sin. 2020;41:670–7.
Park HJ, Shim HS, Kim JY, Kim JY, Park SK, Shim I. Ginseng purified dry extract, BST204, improved cancer chemotherapy-related fatigue and toxicity in mice. Evid Based Complement Altern Med. 2015;2015:197459.
Dougherty JP, Wolff BS, Cullen MJ, Saligan LN, Gershengorn MC. Taltirelin alleviates fatigue-like behavior in mouse models of cancer-related fatigue. Pharmacol Res. 2017;124:1–8.
Han YH, Mun JG, Jeon HD, Yoon DH, Choi BM, Kee JY, et al. The extract of Arctium lappa L. fruit (Arctii Fructus) improves cancer-induced cachexia by inhibiting weight loss of skeletal muscle and adipose tissue. Nutrients. 2020;12:3195.
Yang F, Zhou L, Song J, WangJinMei A, Yang Y, Tang ZW, et al. Liver CEBPβ modulates the kynurenine metabolism and mediates the motility for hypoxia-induced central fatigue in mice. Front Physiol. 2019;10:243.
Wang X, Qu Y, Zhang Y, Li S, Sun Y, Chen Z, et al. Antifatigue potential activity of sarcodon imbricatus in acute excise-treated and chronic fatigue syndrome in mice via regulation of Nrf2-mediated oxidative stress. Oxid Med Cell Longev. 2018;2018:9140896.
Zhang X, Jing S, Lin H, Sun W, Jiang W, Yu C. et al. Anti-fatigue effect of anwulignan via the NRF2 and PGC-1α signaling pathway in mice. Food Funct. 2019;10:7755–66.
Zhou YT, Li SS, Ai M, Chen H, Liu YX, Li BY, et al. 1,25(OH)2D3 mitigate cancer-related fatigue in tumor-bearing mice: Integrating network pharmacological analysis. Biomed Pharmacother. 2020;128:110256.
Zhang Y, Li Z, Zhao Z, Kuai W, Wei C, Lv J, et al. Effect of the chinese medicine YangZheng XiaoJi on reducing fatigue in mice with orthotopic transplantation of colon cancer. Evid Based Complement Altern Med. 2019;2019:3870812.
Minton O, Alexander S, Stone PC. Identification of factors associated with cancer related fatigue syndrome in disease-free breast cancer patients after completing primary treatment. Breast Cancer Res Treat. 2012;136:513–20.
Gill BS, Sharma P, Kumar R, Kumar S. Misconstrued versatility of Ganoderma lucidum: a key player in multi-targeted cellular signaling. Tumour Biol. 2016;37:2789–804.
Powers SK, Lynch GS, Murphy KT, Reid MB, Zijdewind I. Disease-induced skeletal muscle atrophy and fatigue. Med Sci Sports Exerc. 2016;48:2307–19.
Grossberg AJ, Vichaya EG, Christian DL, Molkentine JM, Vermeer DW, Gross PS, et al. Tumor-associated fatigue in cancer patients develops independently of IL1 signaling. Cancer Res. 2018;78:695–705.
Rutherford G, Manning P, Newton JL. Understanding muscle dysfunction in chronic fatigue syndrome. J Aging Res. 2016;2016:2497348.
Shen Q, Miao C-X, Zhang W-L, Li Y-W, Chen Q-Q, Li X-X, et al. SiBaoChongCao exhibited anti-fatigue activities and ameliorated cancer cachexia in mice. RSC Adv. 2019;9:17440–56.
Niere M, Kernstock S, Koch-Nolte F, Ziegler M. Functional localization of two poly(ADP-ribose)-degrading enzymes to the mitochondrial matrix. Mol Cell Biol. 2008;28:814–24.
Liu R, Wu L, Du Q, Ren JW, Chen QH, Li D, et al. Small molecule oligopeptides isolated from Walnut (Juglans regia L.) and their anti-fatigue effects in mice. Molecules. 2018;24:45.
Li Y, Xin Y, Xu F, Zheng M, Xi X, Cui X, et al. Maca polysaccharides: extraction optimization, structural features and anti-fatigue activities. Int J Biol Macromol. 2018;115:618–24.
Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–85.
Hardee JP, Montalvo RN, Carson JA. Linking cancer cachexia-induced anabolic resistance to skeletal muscle oxidative metabolism. Oxid Med Cell Longev. 2017;2017:8018197.
Carson JA, Hardee JP, VanderVeen BN. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Semin Cell Dev Biol. 2016;54:53–67.
VanderVeen BN, Fix DK, Carson JA. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: a role for inflammation. Oxid Med Cell Longev. 2017;2017:3292087.
White JP, Puppa MJ, Gao S, Sato S, Welle SL, Carson JA. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metab. 2013;304:E1042–52.
Wong J, Smith LB, Magun EA, Engstrom T, Kelley-Howard K, Jandhyala DM, et al. Small molecule kinase inhibitors block the ZAK-dependent inflammatory effects of doxorubicin. Cancer Biol Ther. 2013;14:56–63.
Wood LJ, Weymann K. Inflammation and neural signaling: etiologic mechanisms of the cancer treatment-related symptom cluster. Curr Opin Support Palliat Care. 2013;7:54–9.
Casaril AM, Domingues M, Bampi SR, Lourenço DA, Smaniotto T, Segatto N, et al. The antioxidant and immunomodulatory compound 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole attenuates depression-like behavior and cognitive impairment developed in a mouse model of breast tumor. Brain Behav Immun. 2020;84:229–41.
Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends Neurosci. 2014;37:39–46.
Morris G, Maes M. Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Curr Neuropharmacol. 2014;12:168–85.
Shui L, Yi RN, Wu YJ, Bai SM, Si Q, Bo AG, et al. Effects of mongolian marm acupuncture on iNOS/NO and inflammatory cytokines in the hippocampus of chronic fatigue rats. Front Integr Neurosci. 2019;13:78.
Yuan R, Huang L, Du LJ, Feng JF, Li J, Luo YY, et al. Dihydrotanshinone exhibits an anti-inflammatory effect in vitro and in vivo through blocking TLR4 dimerization. Pharmacol Res. 2019;142:102–14.
Liu FY, Cai J, Wang C, Ruan W, Guan GP, Pan HZ, et al. Fluoxetine attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage: a possible role for the regulation of TLR4/MyD88/NF-κB signaling pathway. J Neuroinflammation. 2018;15:347.
Cleary JM, Mamon HJ, Szymonifka J, Bueno R, Choi N, Donahue DM, et al. Neoadjuvant irinotecan, cisplatin, and concurrent radiation therapy with celecoxib for patients with locally advanced esophageal cancer. BMC Cancer. 2016;16:468.
Norden DM, McCarthy DO, Bicer S, Devine RD, Reiser PJ, Godbout JP, et al. Ibuprofen ameliorates fatigue- and depressive-like behavior in tumor-bearing mice. Life Sci. 2015;143:65–70.
Zhu X, Burfeind KG, Michaelis KA, Braun TP, Olson B, Pelz KR, et al. MyD88 signalling is critical in the development of pancreatic cancer cachexia. J Cachexia Sarcopenia Muscle. 2019;10:378–90.
Chen MC, Hsu WL, Hwang PA, Chen YL, Chou TC. Combined administration of fucoidan ameliorates tumor and chemotherapy-induced skeletal muscle atrophy in bladder cancer-bearing mice. Oncotarget. 2016;7:51608–18.
Lee H, Heo JW, Kim AR, Kweon M, Nam S, Lim JS, et al. Z-ajoene from crushed garlic alleviates cancer-induced skeletal muscle atrophy. Nutrients. 2019;11:2724.
Irwin MR. Inflammation at the intersection of behavior and somatic symptoms. Psychiatr Clin North Am. 2011;34:605–20.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grants 81620108029, 81974083, 81330074, 81500535 and 81800388), the Beijing Natural Science Foundation (grant 7212151), and the China Postdoctoral Science Foundation (grant 2018M630049).
Author information
Authors and Affiliations
Contributions
AA, BXY, and ML conceptualized and designed this study. AA, LH, AM, FYS, HZZ, SML, GYS, YX, JHR, JL, DML, and LFW performed the experiments and analyzed the data. AA and BXY wrote the paper. HZ, ML, and BXY reviewed and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Abulizi, A., Hu, L., Ma, A. et al. Ganoderic acid alleviates chemotherapy-induced fatigue in mice bearing colon tumor. Acta Pharmacol Sin 42, 1703–1713 (2021). https://doi.org/10.1038/s41401-021-00669-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00669-6
Keywords
This article is cited by
-
Bcl-xL DNAzymes promote radiosensitivity and chemosensitivity in colorectal cancer cells via enhancing apoptosis
BMC Pharmacology and Toxicology (2022)