Abstract
Dry eye disease (DED) is a multifactorial disorder of the tears and ocular surface characterized by manifestations of dryness and irritation. Although the pathogenesis is not fully illuminated, it is recognized that inflammation has a prominent role in the development and deterioration of DED. β-aminoarteether maleate (SM934) is a water-soluble artemisinin derivative with anti-inflammatory and immunosuppressive activities. In this study, we established scopolamine hydrobromide (SCOP)-induced rodent model as well as benzalkonium chloride (BAC)-induced rat model to investigate the therapeutic potential of SM934 for DED. We showed that topical application of SM934 (0.1%, 0.5%) significantly increased tear secretion, maintained the number of conjunctival goblet cells, reduced corneal damage, and decreased the levels of inflammatory mediators (TNF-α, IL-6, IL-10, or IL-1β) in conjunctiva in SCOP-induced and BAC-induced DED models. Moreover, SM934 treatment reduced the accumulation of TLR4-expressing macrophages in conjunctiva, and suppressed the expression of inflammasome components, i.e., myeloid differentiation factor88 (MyD88), Nod-like receptor protein 3 (NLRP3), apoptosis-associated speck-like protein containing CARD (ASC), and cleaved caspase 1. In LPS-treated RAW 264.7 cells, we demonstrated that pretreatment with SM934 (10 μM) impeded the upregulation of TLR4 and downstream NF-κB/NLRP3 signaling proteins. Collectively, artemisinin analog SM934 exerts therapeutic benefits on DED by simultaneously reserving the structural integrity of ocular surface and preventing the corneal and conjunctival inflammation, suggested a further application of SM934 in ophthalmic therapy, especially for DED.
Similar content being viewed by others
Introduction
Dry eye disease (DED) is a growing public health concern that causes ocular discomfort and visual disturbance, which interferes with quality of life [1]. The prevalence of DED ranges from 5% to 50%, with a higher morbidity for women than men, according to global epidemiological studies [2]. High osmotic pressure, corneal inflammation and injury, and neurosensory abnormalities can all contribute to the occurrence of DED [3], among which evaporative water loss is considered to be the central mechanism of DED pathogenesis. Although the etiology of DED is complex, either aqueous deficiency or excessive evaporation could induce a self-perpetuating cycle that leads to tear film impairment and ocular surface inflammation, resulting in a loss of the goblet cells that secrete gel-forming mucins and in the death of corneal epithelial cells [4]. Therefore, current treatment strategies for DED mainly include increasing tear fluid and anti-inflammatory treatment.
The Toll-like receptor (TLR) family is a highly conserved family of glycoprotein receptors that is a part of the body’s natural immune system; the receptors are able to recognize patterns of conserved molecular motifs associated with microbial pathogens [5, 6]. The tear film, glycocalyx, and epithelial cells constitute a physical barrier between the eye and the external environment, preventing the adhesion of microorganisms and the invasion of toxins to protect the ocular surface. When the barrier function of the ocular surface is destroyed, the expression of TLRs increases significantly, which activates the NF-κB and MAPK cascades through MyD88-dependent pathways, inducing the secretion of inflammatory factors such as IL-1β, IL-2, IL-9, and TNF-α to recruit immune cells and promote their infiltration [7]. In addition, the upregulation of IL-1 and TNF-α can also enhance the expression of cytokines and inflammatory factors mediated by TLRs’ recognition of natural ligands, which could further activate immune cells [8].
SM934 has been identified as an artemisinin derivative exhibiting prominent anti-inflammatory, immunomodulatory, and tissue-protective properties in multiple inflammatory and autoimmune diseases [9,10,11,12,13,14]. Our previous research demonstrated that SM934 could prohibit B-cell activation and plasma cell formation by inhibiting the TLR/MyD88/NF-κB pathway in vivo and in vitro [15]. Our present study reports for the first time that topical application of SM934, an artemisinin derivative, exerts significant therapeutic benefits on DED, which are demonstrated by the improvement of DED manifestations including increasing tear secretion and stabilizing tear film, and the prevention of ocular histopathological progression, such as ocular surface integrity impairment and corneal and conjunctival inflammation. Furthermore, the stability of the SM934 aqueous solution ensures its formulation as an ophthalmic preparation. These findings cast new light on the potential clinical application of SM934 as an ophthalmic medication, which could simultaneously preserve the structural integrity of the ocular surface and prevent corneal and conjunctival inflammation.
Materials and methods
Drugs
SM934 (β-aminoarteether maleate, molecular formula: C21H33NO9) was synthesized from β-hydroxyarteether at Shanghai Institute of Materia Medica. 1H nuclear magnetic resonance (NMR) spectra, 13C NMR spectra, and electrospray ionization mass spectrometry were used to identify the chemical structure [16, 17]. SM934 was dissolved in phosphate-buffered saline (PBS) as a stock solution (pH = 7.4), and the solution was filtered with a 0.22-μm filter membrane. Scopolamine hydrobromide (SCOP), benzalkonium chloride (BAC), and lipopolysaccharide (LPS) were all purchased from Sigma-Aldrich (St. Louis, MO, US). 0.1% sodium hyaluronate (SH) was purchased from Santen Pharmaceutical Co., Ltd (Suzhou, China).
Animals
SD rats and C57/BL6 mice, which were specific pathogen free grade, were purchased from Shanghai Jiesijie Experimental Animal Co., Ltd and Shanghai Lingchang Biological Technology Co., Ltd, respectively. The animals were maintained on standard chow in an air conditioned room under a 12-h dark/light cycle. All experiments and animals were performed according to the guidelines of the Association for Assessment and Accreditation of Laboratory Animals Care International. All procedures were carried out strictly in accordance with animal care and use protocols (2018-03-ZJP-71, 2018-11-ZJP-86, and 2019-09-ZJP-101) approved by the Institutional Animal Care and Use Committee at Shanghai Institute of Materia Medica. Animal care was conducted in accordance with the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research and was provided under the supervision of a health authority-accredited staff member for animal care and management. The ocular surface of all animals was assessed before grouping to exclude individuals with ocular abnormalities.
SCOP-induced dry eye models
Sixty SD female rats (120–150 g) were randomly divided into the normal group, model group, 0.1% SH group, 0.5% SM934 group, 0.1% SM934 group and solvent group, and there were ten rats in each group. Except for the normal group, SCOP (12.5 mg/d) was injected subcutaneously four times a day (9 a.m., 12 a.m., 3 p.m., and 6 p.m.) for 7 days. All drugs were administered topically on the ocular surface at the same time of modeling, with 20 μL administered per eye, four times a day.
Fifty-two C57/BL6 female mice (8–12 weeks) were randomly divided into the normal group, model group, 0.1% SH group, 0.5% SM934 group, 0.1% SM934 group, and solvent group. Except for the normal group, SCOP (0.5 mg/0.2 mL) was injected subcutaneously four times a day (9 a.m., 12 a.m., 3 p.m., and 6 p.m.) and then the sites were left exposed for 10 days. All drugs were administered topically on the ocular surface at the same time of modeling, with 20 μL administered per eye, four times a day.
Measurement of tear production
Tear production was measured using phenol red cotton threads (Tianjin Jingming New Technology Development Co., Ltd., Tianjin, China), and readings were consistently collected at the same time point (9:30 a.m.). The thread was placed on the lower conjunctival fornix at approximately one-third of the lower eyelid distance from the lateral canthus, and it was left there for 15 s. The length of the wet red thread was measured with calipers. Averages were produced from measurements collected from both eyes.
Detection of goblet cells
Sections cut from paraffin-embedded globes were stained with periodic acid-Schiff (PAS) reagent (Servicebio, Wuhan, China). Three different portions of each specimen were randomly selected for counting, and the average was calculated (cells/high-power visual field, 400×) [18].
Corneal fluorescein staining
Sodium fluorescein (Guangzhou Baiyunshan Mingxing Pharmaceutical Co., Ltd, Guangzhou, China) was introduced into the inferior conjunctival sac with a micropipette. Five to seven minutes after three blinks, the test was performed using a slit-lamp biomicroscope (SL-17, KOWA, Aichi, Japan) under cobalt blue light, and a photo was captured and scored. Each cornea was divided into four quadrants that were scored individually. The intensity of corneal fluorescein staining was calculated using a 4-point scale: 0, absent; 1, superficial stippling micropunctate staining with <30 spots; 2, punctate staining with >30 spots, but no diffuse staining; 3, severe diffuse staining, but no positive plaque/patch; and 4, positive fluorescein plaque/patch. The score of the four areas was summed to generate a final grade, which ranged from 0 to 16.
BAC-induced dry eye model
Forty SD male rats (200 g) were randomly divided into a normal group and a BAC group (0.5% BAC-physiological saline solution), and BAC was administered topically twice daily (9 a.m. and 9 p.m.). After 5 days, they were further divided into the model group, 0.5% SM934 group, 0.1% SM934 group, and solvent group according to the ocular surface corresponding index (neovascularization, opacity, conjunctival irritation, rose bengal staining) score. Twenty minutes after establishment of the model, drugs were administered topically on the ocular surface at 3 h intervals; treatment was 20 μL per eye, and it was administered four times a day for 10 days. The experiment lasted 15 days.
Measurement of corneal neovascularization
Corneal neovascularization was examined under general anesthesia and scored with a slit lamp following previously reported methods [19]. Briefly, the cornea was divided into four quadrants, which were scored separately. The neovascularization area was scored as follows: 0, no vessels growing into the clear cornea; 1, vessel growth in one quarter (or less) of the cornea but not zero; 2, vessel growth in one quarter to one half of the cornea; 3, vessel growth in one half to three quarters of the cornea; and 4, vessel growth in three quarters to the entire surface of the cornea. The final score of corneal neovascularization was calculated by summing the scores of the four quadrants (total, 16 points).
Measurement of corneal opacity
The level of corneal opacity was analyzed using the slit lamp according to a previously described method [19] and was scored as follows: 1, scattered or diffuse area with details of the iris visible; 2, easily discernible translucent areas with details of the iris slightly obscured; 3, opalescent areas with no details of the iris visible and the size of pupil barely discernible; and 4, opaque with the iris not visible. The area of opacity was scored as follows: 1, one quarter (or less) of the area opaque but not zero; 2, greater than one quarter of the area opaque but less than one half; 3, greater than one half of the area opaque but less than three quarters; and 4, greater than three quarters and up to the entire area opaque.
Conjunctival irritation score
Conjunctival hyperemia, chemosis, and ocular discharges were observed under a slit lamp [20]. Conjunctival hyperemia was scored as follows: 0 points: normal blood vessels; 1 point: vascular congestion is bright red; 2 points: vascular congestion is dark red, and blood vessels are difficult to distinguish; and 3 points: diffuse hyperemia is purple-red. Conjunctival edema was scored as follows: 0 points: no edema; 1 point: slight edema; 2 points: obvious edema with partial eyelid eversion; 3 points: edema until the eyelid is almost half closed; and 4 points: edema until the eyelid is more than half closed. Conjunctival secretion was scored as follows: 0 points: no secretions; 1: a small amount of secretions; 2: secretions make the eyelids and eyelashes wet or sticky; and 3: secretions make the entire eye area wet or sticky.
Rose bengal staining
Rat eyes were topically treated with 5 μL of 1% rose bengal dye solution (Sigma, 330000, St. Louis, MO, US) [21]. Rats were anesthetized via isoflurane inhalation using a small animal inhalation anesthesia device. After ~1 min, excess dye was manually removed by blinking to observe and score the result under a slit lamp. Rose bengal dye was used to stain the eroded and denuded areas of the corneal and conjunctival epithelium. Staining evaluation was performed according to the following protocol. First, the ocular surface was divided into three areas: the nasal bulbar conjunctiva, cornea, and temporal bulbar conjunctiva. Each compartment was graded as 0 (no damage), 1 (partial damage), 2 (damage in more than half the area), or 3 (damage in the entire area), with a maximum possible score of 9 points.
Cell culture
RAW 264.7 cells, a murine macrophage cell line, were purchased from the American Type Culture Collection (Manassas, VA, US). The cells were cultured in DMEM (Gibco, NY, US) containing 10% fetal bovine serum (HyClone, Logan, UT, US), 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were cultured in a humidified incubator with 5% CO2 at 37 °C.
Quantitative real-time polymerase chain reaction (PCR) analysis
The conjunctiva tissue was first separated; then, two conjunctiva were combined to serve as one sample, and there were two samples per group. Total RNA was extracted using the RNAsimple Total RNA Kit (TIANGEN, Beijing, China), followed by reverse transcription of total RNA to generate cDNA with a ReverTra® Ace qPCR RT Master Mix kit from Toyobo (Osaka, Japan). Subsequently, cDNA was subjected to quantitative real-time PCR experiments with an ABI 7900H real-time PCR system (Life Technologies, NY, US) using a SYBR® Green Real-time PCR Master Mix Kit from Toyobo (Osaka, Japan). A melting curve analysis was performed after amplification to verify the accuracy of the amplicon. The primer sequences used were described in Table 1.
ELISA analysis
Conjunctiva tissue was homogenized with PBS buffer according at a buffer to tissue weight ratio of 9:1, and the supernatant was separated to detect the expression level of cytokines. The levels of TNF-α, IL-6, and monocyte chemoattractant protein-1 (MCP-1) in conjunctiva supernatant were assessed. RAW 264.7 cells were seeded at a concentration of 1 × 106 cells/mL in 24-well culture plates overnight and then were supplemented with LPS (10 μg/mL) and different concentrations of SM934. After incubation for 48 h, the levels of TNF-α, IL-6, and IL-1β in cell culture supernatants were quantified by ELISA kits (BD Biosciences, NJ, US) according to the manufacturer’s protocols.
Immunofluorescence analysis
Immunohistochemistry was performed on paraffin-embedded cornea and conjunctiva sections. The procedure was the same as that used for immunohistochemistry. Cy3-labeled goat anti-rabbit IgG was incubated with the sections at 37 °C for 30 min. Finally, DAPI was used to stain the nuclei (blue), and the sections were analyzed by confocal laser scanning microscopy (FV10i, Olympus, Tokyo, Japan). Antibody information is as follows: anti-rabbit MMP-9 pAb (Servicebio, GB11132, Wuhan, China); anti-rabbit CD68 pAb (Servicebio, GB11067); anti-rabbit TLR4 pAb (Abcam, ab217274, Cambs, UK); and goat anti-rabbit IgG H&L (Cy3®) preadsorbed (Abcam, ab6939).
Immunohistochemistry and quantification
Immunohistochemistry was performed on paraffin-embedded cornea and conjunctiva sections. After deparaffinization and dehydration, antigen retrieval was performed for 10 min using a microwave before peroxidase quenching for 15 min with 3% H2O2 in PBS. Subsequently, the sections were blocked with 5% bovine serum albumin for 30 min and then were incubated with a primary antibody diluted in PBS overnight at 4 °C. Sections were scanned with a slide scanner (SCN400, Leica, Germany). The size of the positively stained area was determined with ImageJ. Antibody information was as follows: anti-rabbit ZO-1 pAb (Abcam, ab96587); anti-rabbit MPO pAb (Servicebio, GB11224); anti-rabbit TLR4 pAb (Abcam, ab217274); anti-rabbit MyD88 pAb (Servicebio, GB11269); and anti-rabbit Nod-like receptor protein 3 (NLRP3) pAb (Novus, NBP2-12446SS, CO, US).
Western blot analysis
Two or three conjunctiva served as one sample, and there were two samples per group; these groups and samples of cells were lysed in SDS buffer containing a protease inhibitor cocktail, which was followed by homogenization in ice-cold water. RAW 264.7 cells were seeded at a concentration of 1.5 × 106 cells/mL in 6-well culture plates overnight. Cells were then stimulated with LPS (100 ng/mL) and were collected at indicated time points (0, 0.5, 1, 3, and 6 h). Either RAW 264.7 cells were pretreated with SM934 (10 μM) for 24 h and then stimulated with LPS (100 ng/mL) for 1 or 6 h to detect specific proteins. Total protein (5–25 μg) was resolved by SDS-PAGE followed by Western blot analysis with the following primary antibodies: anti-rabbit TLR4 pAb (Abcam, ab217274); anti-rabbit TLR4 mAb (CST, 14358S, MA, US); anti-rabbit MyD88 mAb (CST, 4283S); anti-rabbit NF-κB mAb (CST, 4764S); anti-rabbit p-NF-κB mAb (CST, 3033L); anti-rabbit NLRP3 pAb (Novus, NBP2-12446SS); anti-rabbit NLRP3 mAb (CST, 15101S); anti- mouse ASC mAb (Santa Cruz Biotechnology, sc-514414); anti-rabbit cleaved caspase 1 mAb (CST, 89332); anti-mouse β-actin mAb (Abcam, ab49900); goat anti-mouse HRP (Thermo Scientific, 31430, MA, US); and goat anti-rabbit HRP (Bio-Rad, 1705046, CA, US).
Statistical analysis
The data from this research study were analyzed using GraphPad Prism 7.0 software. Statistical analysis was performed using one-way ANOVA, and data are presented as the mean ± SD. P < 0.05 was considered statistically significant.
Results
SM934 increases tear secretion and maintains goblet cells in SCOP-induced DED models
SCOP functions as a pharmacologic inhibitor of cholinergic receptors in the lacrimal gland and therefore decreases aqueous production to establish aqueous-deficient dry eye animal models. We observed a sharp decrease in tear secretion in both rats and mice 3 days after SCOP injection. Application of 0.5% SM934 and 0.1% SM934 significantly increased tear secretion in SCOP-injected rats (Fig. 1a), but only 0.5% SM934 treatment significantly improved tear secretion in SCOP-injected mice compared with the model group (Fig. 1b). Water loss, reduced expression of glycocalyx mucins, and loss of goblet cells secreting gel-forming mucins are hallmarks of DED [22]. This decreased goblet cell density is proportional to the disease severity. Conjunctival PAS staining and statistical results showed that 0.1% SH, 0.5% SM934, and 0.1% SM934 treatment restored the number of goblet cells in the conjunctival epithelium of SCOP-injected rats (Fig. 1c, d). Similar improvements in goblet cell density were observed in SCOP-injected mice (Fig. 1e, f).
After simultaneous modeling and drug administration, rat tear secretion was detected on days 3, 5, and 7, n = 10 (a); mouse tear secretion was detected on days 3, 5, 7 and 10, n = 6–7 (b); at the end of day 7, PAS staining was performed, and rat conjunctival goblet cells (c) and rat goblet cells were counted, n = 10 (d); at the end of day 10, PAS staining was performed, and mouse conjunctival goblet cells (e) and mouse goblet cells were counted, n = 3–4 (f). Data are presented as the mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. model control group on the same day. The results are from one of three independent experiments with similar results.
SM934 protects the ocular surface barrier in SCOP-induced DED models
Areas in which the epithelial cells of the corneal surface are loose or desquamated can be stained with fluorescein dye. As shown in Fig. 2a, b, SCOP-injected rats developed severe corneal defects starting on the third day after injection, and ocular surface epithelium damage was highlighted by corneal fluorescein staining. A much lower fluorescence intensity of the ocular surface was observed in 0.5% SM934- and 0.1% SM934-treated rats. Meanwhile, the expression of the tight junction protein ZO-1 in the corneal epithelium following SM934 treatment was in accordance with the results of corneal fluorescein staining (Fig. 2c, d), indicating that SM934 has a protective effect against ocular surface integrity impairment.
After simultaneous modeling and administration of treatment in rats, fluorescein sodium staining of the cornea was performed on the 3rd, 5th, and 7th days (a) and data were analyzed, n = 10 (b); immunohistochemical staining of ZO-1 in the corneal epithelium of rats, ×400 (c) and positive staining area analysis, n = 5–6 (d); immunofluorescence staining of MMP-9 in the corneal epithelium of rats, ×400 (e) and fluorescence intensity analysis, n = 3 (f); immunofluorescence staining of MMP-9 in the corneal epithelium of mice, ×400 (g) and fluorescence intensity analysis, n = 3 (h). Data are presented as the mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. model control group.
Corneal epithelial cells produce MMPs in response to desiccating stress, and they promote corneal extracellular matrix degradation and epithelial cell loss [23]. Topical application of SM934, especially 0.5% SM934, significantly decreased the elevated MMP-9 levels in the corneal epithelium of SCOP-induced DED in both rats (Fig. 2e, f) and mice (Fig. 2g, h).
SM934 suppresses corneal and conjunctival inflammation in SCOP-induced DED models
Accumulated evidence suggests that inflammation frequently accompanies DED in rodents [24] and humans [25, 26]. Rashid et al. reported that corneal and conjunctival expression of inflammatory cytokines, including IL-1 and TNF-α, was increased in SCOP-induced rodent DED; however, only the conjunctiva showed increased expression of IL-6 and IL-10, and IL-10 was especially elevated, with a 97.6-fold increase in transcripts relative levels in the normal eye [27]. We demonstrated that treatment with 0.5% SM934 for 7 days significantly reduced the expression of NLRP3 and caspase 1 and decreased the levels of TNF-α, IL-1β, IL-6, and IL-10 in the conjunctiva of SCOP-injected rats (Fig. 3a, b).
TNF-α, NLRP3, Caspase 1, and IL-1β mRNA levels in conjunctiva, n = 4–8 (a); conjunctiva homogenate supernatant cytokines TNF-α, IL-6, and IL-10 and monocyte chemokine MCP-1 were analyzed by ELISA, n = 6–8 (b); immunohistochemical staining to detect conjunctiva MPO expression, ×200 (c) and positive staining area analysis, n = 2–3 (d). Data are presented as the mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. model control group.
Moreover, as shown in Fig. 3b, 0.5% SM934 application also suppressed the upregulated monocyte chemoattractant protein-1 (MCP-1) and diminished the accumulation of monocytes, as visualized by MPO staining of the conjunctiva (Fig. 3c).
SM934 ameliorates ocular disruption and alleviates conjunctival inflammation in BAC-induced DED rats
BAC is commonly used as a preservative in ophthalmic preparations, and BAC accumulation can induce instability of tear film and excessive evaporation, which are characteristic features of DED [18, 28]. Typically, application of BAC results in corneal opacity and neovascularization, conjunctival irritation (edema, congestive, and secretions), and inflammatory responses in animal DED models.
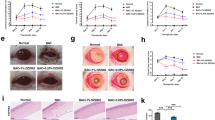
Rats were topically administered 0.5% BAC and subsequently treated with 0.1% SM934, 0.5% SM934 or solvent for 10 consecutive days starting on day 5 after the first application of BAC. As shown in Fig. 4a, topical treatment with SM934, especially 0.5% SM934, ameliorated the severe corneal opacity and neovascularization induced by BAC in DED rats. Furthermore, the changes were evaluated by scoring methods, which revealed significant improvement of BAC-induced corneal opacity (Fig. 4b) and corneal neovascularization following SM934 treatment (Fig. 4c).
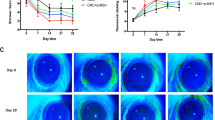
Corneal opacity and neovascularization (a); data analysis is shown for corneal opacity scores on the 4th, 7th, and 10th days after drug intervention (b) and for corneal neovascularization scores (c); data analysis is shown for conjunctival irritation (d) and conjunctival irritation, including hyperemia, edema, total secretion scores (e); corneal rose bengal staining (f) and rose bengal staining score data analysis (g), n = 8; conjunctival homogenate supernatant cytokines TNF-α, IL-6, IL-10, and monocyte chemokine MCP-1 analysis, as shown by ELISA, n = 5–8 (h). Data are presented as the mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. model control group.
Serious conjunctival damage, including conjunctival hyperemia, edema, and secretion, occurred in BAC-irritated rats, and both 0.1% and 0.5% SM934 application significantly alleviated these symptoms 4 days after treatment, as demonstrated by slit-lamp observation (Fig. 4d) and a comprehensive scoring method for evaluating the extent of conjunctival irritation (Fig. 4e).
Further, we used rose bengal dye, which enables easy visualization of the conjunctiva and cornea as a rose pink-colored stain, to examine the extent of ocular disruption. Diffuse red staining was observed in the cornea and conjunctiva of BAC-irritated rats. Both solvent and SM934 application could mitigate the erosion of ocular surfaces, but only 0.1% and 0.5% SM934-treated rats exhibited sustained remission from day 4 after treatment until the end of the experiment (Fig. 4f, g).
As displayed in Fig. 4h, a strong inflammatory response to BAC was observed in the conjunctiva of model rats, and similar to the effects on SCOP-induced DED animals, SM934 application significantly decreased the cytokine levels of TNF-α, IL-6, and IL-10 and the expression of MCP-1 in conjunctival tissues.
Our results demonstrated beneficial effects of topical application of SM934 in treating ocular signs and reversing the inflammatory changes in DED.
SM934 decreases TLR4 expression and abates inflammatory signaling in the conjunctiva of DED rats
DED is an immunoinflammatory disorder of the ocular surface in which tear film instability and hyperosmolarity induce pathogenic responses in the cornea and conjunctiva. Damage-associated molecules and excessive inflammatory cytokines released during tissue injury subsequently activate resident antigen presenting cells (APCs) and promote further immune cell infiltration [29].
It has also been suggested that TLR4 is a receptor for endogenous ligands associated with noninfectious diseases [30, 31]. Lee et al. [32] reported that homogenized DED corneal tissue could activate APCs, and this activation could be abolished by the selective inhibition of TLR4. Regarding the prominent suppressive effects of SM934 on ocular surface conjunctiva inflammation and the protective benefits on corneal epithelial cells, we speculated that TLR4 might play an essential role in the therapeutic effects of SM934 in DED.
Immunofluorescent staining showed that injection with SCOP increased the cell surface expression of TLR4 by CD68-positive macrophages in the conjunctival epithelium of rats, and the accumulation of TLR4-expressing macrophages diminished after treatment with 0.5% SM934 (Fig. 5a). TLR4 ligation triggers the activation of nuclear factor-kappa B (NF-κB) through the assembly of a complex composed of myeloid differentiation primary response 88 (MyD88). TLR4/MyD88/NF-κB signaling in turn upregulates the transcription of proinflammatory factors such as TNF-α and NLRP3, which are components of the inflammasome [33]. Therefore, we sought to evaluate the impact of SM934 treatment on the activation of TLR4 signaling in conjunctival tissue. As shown in Fig. 5b, c, in accordance with the enhanced expression of TLR4, both Myd88 and NLRP3 protein accumulated in the basal layer of conjunctival epithelium in DED rats, and the expression of these proteins was significantly decreased after topical application of SM934. Further, conjunctival tissue homogenates subjected to Western blotting demonstrated that SM934 treatment suppressed the increase in TLR4/Myd88/NF-κB signaling and the subsequent activation of the inflammasome, which consisted of NLRP3, ASC, and cleaved caspase 1 (Fig. 5d, e).
Immunofluorescence costaining of CD68 and TLR4 in conjunctiva, ×600 (a); immunohistochemical staining of conjunctiva TLR4, MyD88, and NLRP3, ×400 (b) and analysis of the positively stained area, n = 3-5 (c); Western blot assay of indicated proteins in the conjunctival homogenates (d) and densitometry analyses of the Western blotting results, n = 2–4 (e). Data are presented as the mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. the model control group.
SM934 impedes the upregulation of TLR4 and downstream signal transduction in LPS-elicited RAW 264.7 cells
Previous studies [34, 35] found that exposure to desiccating stress conditions skewed corneal and conjunctival resident macrophage polarization toward an inflammatory phenotype. Regarding the observation of remarkable accumulation of TLR4-expressing macrophages and increased levels of TNF-α and IL-6 in conjunctiva of the DED model, which were significantly attenuated by SM934 treatment, we hypothesized that topical application of SM934 could constrain focal inflammation by inhibiting TLR4 signaling in macrophages.
To gain experimental evidence regarding the therapeutic benefits of SM934 on DED, we conducted cell culture studies using the murine macrophage cell line RAW 264.7; we challenged the cells with LPS, a typical TLR4 agonist. In line with the results from conjunctival tissue in both SCOP- and BAC-induced DED rats, cells incubated with SM934 produced lower levels of the inflammatory cytokines IL-6, TNF-α, and IL-1β following 48 h of LPS stimulation compared with cells conditioned with LPS only (Fig. 6a).
The levels of TNF-α, IL-6, and IL-1β in the supernatant of LPS (10 μg/mL)-induced RAW 264.7 cells were analyzed by ELISA to assess the inflammatory response after 48 h (a); Western blot assay of the indicated proteins in LPS (100 ng/mL)-stimulated RAW 264.7 cells at different time points (b) and densitometry analyses of the immunoblotting results (c); Western blot assay of the indicated proteins in SM934 pretreated RAW 264.7 cells followed by LPS (100 ng/mL) stimulation for 1 h (TLR4, MyD88, NF-κB, and p-NF-κB) and 6 h (NLRP3, ASC, and cleaved caspase 1) (d) and the densitometry analyses of the immunoblotting results (e). Data are presented as the mean ± SD. ##P < 0.01, ###P < 0.001 vs. the 0-h time point; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. the LPS-treated group. The results are derived from at least three independent experiments.
Furthermore, as shown in Fig. 6b, c, macrophages exhibited an upregulation of TLR4 expression 0.5–1 h after LPS exposure, which was followed by a pronounced phosphorylation of NF-κB from 0.5 to 6 h after stimulation. A dramatic NLRP3 priming signal could be monitored 2.5 h after NF-κB activation, indicating subsequent inflammasome activation. In light of the dose-dependent inhibitory effects of SM934 on inflammatory mediator production, we pretreated RAW 264.7 cells with 10 μM SM934 for 24 h before LPS stimulation and found that SM934 significantly prevented the upregulation of TLR4 expression and impeded NF-κB and NLRP3 activation (Fig. 6d, e).
Discussion
DED is a common and multifactorial disease with a high prevalence worldwide. Water loss, reduced expression of glycocalyx mucins, and loss of goblet cells secreting gel-forming mucins are hallmarks of DED [22].
Scopolamine hydrobromide is an M-type cholinergic receptor blocker that can inhibit tear secretion by inhibiting acetylcholine-mediated tear gland stimulation [36]. BAC is a cationic surfactant that is commonly used as a preservative in ophthalmic preparations [37]. It can promote corneal epithelial cell death by lysing cell membranes, which disrupt corneal epithelial cell connections [38, 39]. Thus, both SCOP and BAC can be applied to establish a DED model [40, 41]. Our results demonstrated that SM934 increased tear secretion, maintained the number of conjunctival goblet cells, and improved corneal and conjunctival damage in DED rat and mouse models induced by SCOP. Moreover, SM934 treatment resulted in the desired improvement in neovascularization, corneal opacity, conjunctival irritation, and corneal damage in DED rats induced by BAC.
The tear film covers the ocular surface and is exposed to the external environment, and mucus gel is the first defense barrier at the ocular surface. The mucins and aqueous layers together form the mucoaqueous layer, with a mucin gradient increasing toward the lipid layer of the ocular surface [42]. A common feature of all forms of DED is the decrease of goblet cell density, which is accompanied by a decline of the gel-forming mucin and is related to deterioration of the disease. Once mucin production has been disrupted, subsequent exposure of ocular surface epithelial cells to tear hyperosmolarity activates a stress-associated signaling pathway that initiates the production of proinflammatory cytokines, chemokines, and MMPs. In our study, topical administration of SM934 on the ocular surface significantly preserved the number of goblet cells in the conjunctiva, maintained the secretion of tear, and promoted the integrity of tear film in DED induced by SCOP or BAC. Moreover, SM934 treatment reduced the expression of MMP-9 in the corneal epithelium of DED rats; MMP-9 is a critical metalloproteinase produced by corneal epithelial cells in response to hyperosmolar stress, so reducing its levels ameliorated corneal extracellular matrix degradation and epithelial cell loss.
The proinflammatory milieu in DED facilitates the activation and maturation of APCs, and the matured APCs migrate through the afferent lymphatics and efferent blood vessels to the ocular surface. Microscopy revealed that in the corneas of mice with DED, there is evidence of an influx of CD11b+ APCs, including dendritic cells and macrophages [43, 44]. In our study, we observed a pronounced accumulation of CD68+ macrophages in the conjunctiva of DED rats, and they exhibited increased expression of TLR4 on their cell surface. As reported by Lee et al. [32], the altered expression pattern of TLR4 and the excessive production of endogenous TLR4 ligands, such as extracellular matrix degradation products, might contribute to the subsequent activation and migration of APCs in corneal tissue in DED. We hypothesized that SM934 suppressed the elevation of TLR4 expression and prevented MMP-9-mediated tissue degradation of the ocular surface, hence alleviating corneal injury and conjunctival inflammation in DED models.
Upon binding to its ligands, TLR4 signaling triggers the assembly of a complex containing MyD88, stimulates the release of NF-κB from its repressor IκB, and initiates the transcription of proinflammatory factors, such as TNF-α, and inflammasome components, such as pro-IL-1β and NLRP3. Inflammasome activation involves assembly of NLRP3 with ASC and pro-caspase 1. This complex triggers pro-caspase 1 self-cleavage into active caspase 1, which then cleaves pro-IL-1β to generate biologically active cytokine IL-1β [33]. Intriguingly, we found in LPS-elicited macrophages that SM934 treatment not only could downregulate TLR4 expression induced by LPS stimulation but also could prohibit the downstream activation of inflammasomes and the production of proinflammatory cytokines TNF-α, IL-1β, and IL-6. These findings provide fundamental support for the mechanism of the anti-inflammatory and tissue-protective effects of SM934 on DED.
SM934 is a novel water-soluble artemisinin analog that possesses desirable anti-inflammatory and immunomodulatory activities. In addition to its pharmacological effects, the stability and solubility of SM934 in aqueous solution further ensure its applicable potential as an ophthalmic remedy for DED.
Conclusion
In summary, our research demonstrated for the first time that topical administration of SM934 significantly ameliorated SCOP and BAC-induced DED in rodent models. The mechanism of therapeutic benefits might be related to its direct inhibitory effect on the TLR4/NF-κB/NLRP3 inflammatory pathway in macrophages, as well as the comprehensive modulation of aberrant TLR4 expression and the generation of endogenous TLR4 ligands in ocular tissues during DED.
References
Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. 2013;1:51–7.
Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334–65.
Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83.
Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2019;17:842.
Peri F, Piazza M. Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists. Biotechnol Adv. 2012;30:251–60.
Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–74.
Reins RY, Lema C, Courson J, Kunnen CME, Redfern RL. MyD88 deficiency protects against dry eye-induced damage. Invest Ophthalmol Vis Sci. 2018;59:2967–76.
Simmons KT, Xiao YY, Pflugfelder SC, de Paiva CS. Inflammatory response to lipopolysaccharide on the ocular surface in a murine dry eye model. Invest Ophth Vis Sci. 2016;57:2443–51.
Hou LF, He SJ, Li X, Wan CP, Yang Y, Zhang XH, et al. SM934 treated lupus-prone NZB x NZW F1 mice by enhancing macrophage interleukin-10 production and suppressing pathogenic T cell development. PLoS One. 2012;7:e32424.
Hou LF, He SJ, Li X, Yang Y, He PL, Zhou Y, et al. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum. 2011;63:2445–55.
Yan YX, Shao MJ, Qi Q, Xu YS, Yang XQ, Zhu FH, et al. Artemisinin analogue SM934 ameliorates DSS-induced mouse ulcerative colitis via suppressing neutrophils and macrophages. Acta Pharmacol Sin. 2018;39:1633–44.
Li TT, Zhang XH, Jing JF, Li X, Yang XQ, Zhu FH, et al. Artemisinin analogue SM934 ameliorates the proteinuria and renal fibrosis in rat experimental membranous nephropathy. Acta Pharmacol Sin. 2015;36:188–99.
Li X, Li TT, Zhang XH, Hou LF, Yang XQ, Zhu FH, et al. Artemisinin analogue SM934 ameliorates murine experimental autoimmune encephalomyelitis through enhancing the expansion and functions of regulatory T cell. PLoS One. 2013;8:e74108.
Lin ZM, Yang XQ, Zhu FH, He SJ, Tang W, Zuo JP. Artemisinin analogue SM934 attenuate collagen-induced arthritis by suppressing T follicular helper cells and T helper 17 cells. Sci Rep. 2016;6:38115.
Wu Y, He S, Bai B, Zhang L, Xue L, Lin Z, et al. Therapeutic effects of the artemisinin analog SM934 on lupus-prone MRL/lpr mice via inhibition of TLR-triggered B-cell activation and plasma cell formation. Cell Mol Immunol. 2016;13:379–90.
Yang ZS, Wang JX, Zhou Y, Zuo HP, Li Y. Synthesis and immunosuppressive activity of new artemisinin derivatives. Part 2: 2-[12(beta or alpha)-dihydroartemisinoxymethyl-(or 1'-ethyl)]phenoxyl propionic acids and esters. Bioorgan Med Chem. 2006;14:8043–9.
Yang ZS, Zhou WL, Sui Y, Wang JX, Wu JM, Zhou Y. et al. Synthesis and immunosuppressive activity of new artemisinin derivatives. 1. [12(beta or alpha)-dihydroartemisininoxylphen(ox)yl aliphatic acids and esters. J Med Chem. 2005;48:4608–17.
Yang Q, Zhang YF, Liu XP, Wang N, Song ZY, Wu KL. A comparison of the effects of benzalkonium chloride on ocular surfaces between C57BL/6 and BALB/c mice. Int J Mol Sci. 2017;18:509. https://doi.org/10.3390/ijms18030509.
Pauly A, Brignole-Baudouin F, Labbe A, Liang H, Warnet JM, Baudouin C. New tools for the evaluation of toxic ocular surface changes in the rat. Invest Ophthalmol Vis Sci. 2007;48:5473–83.
Nakatani H, Gomes P, Bradford R, Guo Q, Safyan E, Hollander DA. Alcaftadine 0.25% versus olopatadine 0.1% in preventing cedar pollen allergic conjunctivitis in Japan: a randomized study. Ocul Immunol Inflamm. 2019;27:622–31.
Na YJ, Choi KJ, Park SB, Sung HR, Jung WH, Kim HY, et al. Protective effects of carbenoxolone, an 11beta-HSD1 inhibitor, against chemical induced dry eye syndrome. Apoptosis. 2017;22:1441–53.
Portal C, Gouyer V, Gottrand F, Desseyn JL. Ocular mucins in dry eye disease. Exp Eye Res. 2019;186:107724.
Corrales RM, Stern ME, De Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:3293–302.
Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–301.
Gulati A, Sacchetti M, Bonini S, Dana R. Chemokine receptor CCR5 expression in conjunctival epithelium of patients with dry eye syndrome. Arch Ophthalmol. 2006;124:710–6.
Mantelli F, Mauris J, Argueso P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr Opin Allergy Clin Immunol. 2013;13:563–8.
Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–25.
Lemp MA, Baudouin C, Baum J, Dogru M, Foulks GN, Kinoshita S, et al. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the international Dry Eye WorkShop. Ocul Surf. 2007;5:75–92.
Lema C, Reins RY, Redfern RL. High-mobility group box 1 in dry eye inflammation. Invest Ophthalmol Vis Sci. 2018;59:1741–50.
Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, et al. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:I270–4.
Kerfoot SM, Long EM, Hickey MJ, Andonegui G, Lapointe BM, Zanardo RC, et al. TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J Immunol. 2004;173:7070–7.
Lee HS, Hattori T, Park EY, Stevenson W, Chauhan SK, Dana R. Expression of Toll-like receptor 4 contributes to corneal inflammation in experimental dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:5632–40.
Boursereau R, Abou-Samra M, Lecompte S, Noel L, Brichard SM. Downregulation of the NLRP3 inflammasome by adiponectin rescues Duchenne muscular dystrophy. BMC Biol. 2018;16:33. https://doi.org/10.1186/s12915-018-0501-z.
Stout RD, Jiang CC, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–9.
Lee HS, Amouzegar A, Dana R. Kinetics of corneal antigen presenting cells in experimental dry eye disease. BMJ Open Ophthalmol. 2017;1:e000078.
Chen Y, Chauhan SK, Lee HS, Stevenson W, Schaumburg CS, Sadrai Z, et al. Effect of desiccating environmental stress versus systemic muscarinic AChR blockade on dry eye immunopathogenesis. Invest Ophthalmol Vis Sci. 2013;54:2457–64.
Olson RJ, White GL Jr. Preservatives in ophthalmic topical medications: a significant cause of disease. Cornea. 1990;9:363–4.
Rasmussen CA, Kaufman PL, Kiland JA. Benzalkonium chloride and glaucoma. J Ocul Pharmacol Ther. 2014;30:163–9.
Datta S, Baudouin C, Brignole-Baudouin F, Denoyer A, Cortopassi GA. The eye drop preservative benzalkonium chloride potently induces mitochondrial dysfunction and preferentially affects LHON mutant cells. Invest Ophthalmol Vis Sci. 2017;58:2406–12.
Ru Y, Huang Y, Liu H, Du J, Meng Z, Dou Z, et al. Alpha-Melanocyte-stimulating hormone ameliorates ocular surface dysfunctions and lesions in a scopolamine-induced dry eye model via PKA-CREB and MEK-Erk pathways. Sci Rep. 2015;5:18619. https://doi.org/10.1038/srep18619.
Droy-Lefaix MT, Bueno L, Caron P, Belot E, Roche O. Ocular inflammation and corneal permeability alteration by benzalkonium chloride in rats: a protective effect of a myosin light chain kinase inhibitor. Invest Ophthalmol Vis Sci. 2013;54:2705–10.
Willcox MDP, Argueso P, Georgiev GA, Holopainen JM, Laurie GW, Millar TJ, et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15:366–403.
Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90–100.
Lin H, Li W, Dong N, Chen W, Liu J, Chen L, et al. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci. 2010;51:122–8.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 81871240); the National Science and Technology Major Project “New Drug Creation and Manufacturing Program,” China (Grant No. 2018ZX09711002-014-001); the Personalized Medicines—Molecular Signature-based “Drug Discovery and Development,” Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA12020107 and XDA12020369).
Author information
Authors and Affiliations
Contributions
FMY, SJH, and JPZ contributed to the conception and design of this study; FMY, XQY, and FHZ completed the main part of the experiment; MJS, DF, and QL participated in the in vivo experiment; YTL, ZML, and SQC participated in the in vitro experiment; JPZ, WT, and SJH contributed to drafting the manuscript and revising it critically for important intellectual content; and all authors contributed to this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Yang, Fm., Fan, D., Yang, Xq. et al. The artemisinin analog SM934 alleviates dry eye disease in rodent models by regulating TLR4/NF-κB/NLRP3 signaling. Acta Pharmacol Sin 42, 593–603 (2021). https://doi.org/10.1038/s41401-020-0484-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-0484-5
Keywords
This article is cited by
-
DZ2002 alleviates corneal angiogenesis and inflammation in rodent models of dry eye disease via regulating STAT3-PI3K-Akt-NF-κB pathway
Acta Pharmacologica Sinica (2024)
-
Aurantio-obtusin Alleviates Dry Eye Disease by Targeting NF-κB/NLRP3 Signaling in Rodent Models
Biochemical Genetics (2024)
-
Artemisinin derivative SM934 in the treatment of autoimmune and inflammatory diseases: therapeutic effects and molecular mechanisms
Acta Pharmacologica Sinica (2022)
-
Discovery and repurposing of artemisinin
Frontiers of Medicine (2022)