Abstract
Disrupted-in-schizophrenia 1 (DISC1) is a scaffold protein that has been implicated in multiple mental disorders. DISC1 is known to regulate neuronal proliferation, signaling, and intracellular calcium homeostasis, as well as neurodevelopment. Although DISC1 was linked to sleep-associated behaviors, whether DISC1 functions in the circadian rhythm has not been determined yet. In this work, we revealed that Disc1 expression exhibits daily oscillating pattern and is regulated by binding of circadian locomotor output cycles kaput (CLOCK) and Brain and muscle Arnt-like protein-1 (BMAL1) heterodimer to E-box sequences in its promoter. Interestingly, Disc1 deficiency increases the ubiquitination of BMAL1 and de-stabilizes it, thereby reducing its protein levels. DISC1 inhibits the activity of GSK3β, which promotes BMAL1 ubiquitination, suggesting that DISC1 regulates BMAL1 stability by inhibiting its ubiquitination. Moreover, Disc1-deficient cells and mice show reduced expression of other circadian genes. Finally, Disc1-LI (Disc1 knockout) mice exhibit damped circadian physiology and behaviors. Collectively, these findings demonstrate that the oscillation of DISC1 expression is under the control of CLOCK and BMAL1, and that DISC1 contributes to the core circadian system by regulating BMAL1 stability.
Similar content being viewed by others
Introduction
Anticipating the time of day is crucial for organisms that live under recurrent sunlight. The molecular circadian clock system maintains the daily cycle of organisms1,2,3,4,5. Transcription–translation feedback loop (TTFL) of circadian genes ensures this cycle, which keeps the circadian clock ticking even without external time cues5. In the mammalian TTFL system, the main component is the heterodimer consisting of Circadian locomotor output cycles kaput (CLOCK) and Brain and muscle Arnt-like protein-1 (BMAL1), which binds to E-box sequences of target genes for transcriptional enhancement6,7. In this way, the CLOCK/BMAL1 heterodimer upregulates core clock genes, including Period (PERs), Cryptochrome (CRYs), and Nuclear receptor subfamily 1 (Nr1d1)5. In turn, the action of CLOCK/BMAL1 heterodimer is negatively regulated by PERs and CRYs, thus completing the TTFL8,9.
Timed regulation of circadian proteins is essential to the proper working of the circadian timing system. To this end, the degradation of the molecular circadian components is vital. Newly synthesized circadian proteins must be degraded at the right time for the accurate pacing of the circadian clock. Indeed, several circadian components, including BMAL1, PERs, CRYs, and REV-ERBs, are regulated by degradation pathways10. Glycogen synthase kinase 3β (GSK3β) phosphorylates BMAL1, thereby enhancing its ubiquitination and degradation11. Moreover, casein kinases Iδ/ε (CKIδ/ε) phosphorylate PERs leading to their degradation10. Chemical inhibition of CKI augments the stability of PERs and induces a more extended circadian period. F-box and leucine-rich repeat protein 3 (FBXL3), an E3 ubiquitin ligase, mediates ubiquitination and subsequent degradation of CRYs12, and FBXL3-mutated mice also exhibit longer circadian period13. REV-ERBα is phosphorylated by cyclin-dependent kinase 1 (CDK1) and recognized for ubiquitination by F-box and WD repeat domain containing 7 (FBXW7)14. The loss-of-function of FBXW7 results in a damped circadian amplitude, indicating that the degradation of REV-ERBα is essential for the circadian amplitude. Altogether, these studies indicate that proper degradation of circadian proteins is a critical factor to maintain circadian period and amplitude.
Disrupted-in-schizophrenia 1 (DISC1) was first reported as a responsible gene for prevalent psychiatric conditions, including schizophrenia, in a Scottish pedigree15. Although DISC1 as a genetic risk factor for schizophrenia has more to be elucidated, DISC1 plays significant molecular roles, including functions in early brain development, which are important to the molecular basis of psychiatric disorders16. DISC1 is a scaffold protein with a large number of interacting partners that perform various functions in the nervous system, such as neuronal migration, neurite outgrowth, spine regulation, and synapse maintenance17,18,19,20. Interestingly, DISC1 is associated with sleep-related phenotypes. Expression of human DISC1 in fruit flies alters their sleep homeostasis21. Furthermore, a mouse model of DISC1 gain-of-function shows increased wakefulness and decreased REM and NREM sleep22. A close relationship between sleep and the circadian clock has been proposed23; however, potential direct involvement of DISC1 in the mammalian circadian system has not been explored yet. In this study, we investigated the relationship between DISC1 and the circadian system and proposed a modulatory role of DISC1 in the mammalian molecular clock.
Materials and methods
Animals
All animal-related experiments were approved by Pohang University of Science and Technology Institutional Animal Care and Use Committee (POSTECH-2019-0024 and POSTECH-2019-0025), and animal experiments were conducted with approved guidelines. Wild type C57BL/6J mice were purchased from Hyochang Science (Daegu, South Korea). Disc1 knockout mouse, named as Disc1 locus impairment mouse (Disc1-LI mouse, C57BL/6 J backgrounded), was previously described24 and a kind gift from Dr. Akira Sawa, Johns Hopkins University School of Medicine. In Disc1-LI mouse, exons from 1 to 3 of Disc1 were replaced to Neomycin resistance cassette by homologous recombination24,25. Male wild type (C57BL/6J) mice and Disc1-LI mice were housed under 12-h light 12-h dark conditions with ad libitum access to food and water. Male mice aged 3 to 4 months were employed for circadian experiments.
Cell culture and transfection
HEK293 cell line, NIH3T3 cell line, and Mouse embryonic fibroblasts (MEFs) were maintained in 5% CO2 incubator at 37 °C with DMEM high glucose (Welgene, LM 001-05) containing 10% fetal bovine serum (Merck, ES009B-KC) and 1% penicillin/streptomycin (HyClone, SV30010). Cells were transfected with Polyethylenimine (PEI, Polysciences, Inc., 23966-2) as previously described26. Briefly, plasmids were mixed with PEI (1 mg/ml, pH 7.0) in opti-MEM (Gibco, 31985-070) and added to cells after 15 min of incubation.
Antibodies
For antibodies, rabbit anti-DISC1 (Millipore, ABN-425) was purchased from Millipore; rabbit anti-BMAL1 (Abcam, ab93806) from Abcam; mouse anti-alpha-tubulin (Proteintech, 66031-1-lg) from Proteintech; rabbit anti-HA (Bethyl, A190-108A) from Bethyl; rabbit anti-GFP (Invitrogen, A11122) from Invitrogen; rabbit anti-Flag (ThermoFisher, PA1-984B) from ThermoFisher; mouse anti-Flag (Sigma, F1804) from Sigma; mouse anti-c-Myc (Santa Cruz, sc-40) from Santa Cruz; GSK3β p-Y216 (Invitrogen, 44-604G) from Invitrogen; mouse anti-GSK3β (Cell Signaling, 9832S) from Cell Signaling. Antibodies were utilized as the first antibody of western blot or precipitating antibody of immunoprecipitation.
Plasmids
Human DISC1 promoter (-982 to +47 relative to TSS27) was cloned in pGL3-basic (Promega), whose luciferase was modified as destabilized with PEST sequence28. For distal, middle, and proximal promoter regions of DISC1, −982 to −624, −623 to −324, and −323 to +47 relative to TSS respectively, were cloned in pGL3-basic with destabilized luciferase. PCR and DpnI-based mutation method was utilized to mutate E-box sequences of DISC1 promoter. Primers were used for the PCR; for mutant E-box (−718 relative to TSS): 5′-TCTATGACCGTACTCTCCTTC-3′ and 5′-GAAGGAGAGTACGGTCATAGA-3′; for mutant E-box (−668 relative to TSS): 5′-AGAGCTACTGTATAGCCCTTC-3′ and 5′-GAAGGGCTATACAGTAGCTCT-3′. Human CLOCK cDNA was cloned in mRFP-C1 with myc tagging at C-terminal. Human BMAL1 cDNA was cloned in pEGFP-C1 (Clontech) and pcDNA3.1 myc-His C (Invitrogen). HA-hDISC1 was a kind gift from Dr. Akira Sawa, Johns Hopkins University School of Medicine, and sub-cloned in pFLAG-CMV2 (Sigma). Human GSK3β cDNA was cloned in pcDNA3.1 myc-His C (Invitrogen). HA-Ubiquitin was a kind gift from Dr. Chin Ha Chung, Seoul National University, Seoul, Korea. pLL3.7 vector was utilized for shRNA knockdown experiments. Target sequence for control shRNA, 5′-ACTACCGTTGTATAGGTG-3′, was previously described29; 5′-GCAGGAGGTCAGCAAGGCCTTG-3′ for human DISC1 shRNA was previously described30.
Western blot
For western blot, cells or brain samples were lysed in Nonidet P-40 buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1% NP40, 5 mM EDTA, 1X protease inhibitor cocktail). Samples were sonicated and centrifuged at 13,000 rpm, 4 °C for 10 min to remove debris. Acquired supernatant was mixed with SDS sampling buffer and denatured at 95 °C for 10 min. Denatured samples were separated with SDS-PAGE gel electrophoresis and transferred to PVDF membrane (Immobilon-PSQ PVDF Membrane, Millipore, ISEQ00010). Membranes were blocked with 5% skim milk and incubated with each appropriate first antibody for overnight at 4 °C, then washed 3 times with TBS-T buffer (20 mM Tris-Cl, pH 7.6, 137.5 mM NaCl, 0.25% Tween 20). Further, membranes were incubated with Horseradish peroxidase (HRP) conjugated second antibody for 1 h, followed by 3 times washing with TBS-T buffer. ECL solution (Bio-rad, 1705061) was applied to membranes, and the band signals were detected by chemiluminescent detector (Azure, c280).
Quantitative RT-PCR
Tri-solution (Bio Science Technology, TS200-001) was used for extracting RNA from cells or brain samples. The RNA concentration was measured by Nanodrop 2000 (Thermo), and 1 μg of RNA was subjected to further experiments. ImProm-II™ Reverse Transcription System (Promega, A3800) and poly T primer were used for reverse transcript of RNA samples. FastStart Universal SYBR Green Master (Rox) (Roche, 04913850001) and StepOnePlus Real-Time PCR System (Applied Biosystems) were utilized to conduct qPCR according to manufacturer’s instructions with primers for each gene:
Period1 F: 5′-GCCAAGAAAGATCCGTCGTCAG-3′
Period1 R: 5′-GGGCTTCTTGTCTCCCACATGGAC-3′ (ref. 31)
Period2 F: 5′-GCTTCTGGTCTGGACTGCAC-3′
Period2 R: 5′-GAGTGTCTGAGGGCTCGTTG-3′ (ref. 32)
Cryptochrome1 F: 5′-GTGGATCAGCTGGGAAGAAG-3′
Cryptochrome1 R: 5′-CACAGGGCAGTAGCAGTGAA-3′ (ref. 33)
Bmal1 F: 5′-GCAGTGCCACTGACTACCAAGA-3′
Bmal1 R: 5′-TCCTGGACATTGCATTGCAT-3′ (ref. 34)
Disc1 F: 5′-GAACAGCAGAAGGCTGGGC-3′
Disc1 R: 5′-GACCTTCCAACACTTCCATGC-3′ (ref. 35)
Tbp F: 5′-GGGAGAATCATGGACCAGAA-3′
Tbp R: 5′-CCGTAAGGCATCATTGGACT-3′ (ref. 36)
Gapdh F: 5′-CACTGAAGGGCATCTTGG-3′
Gapdh R: 5′-TTACTCCTTGGAGGCCATG-3′ (ref. 37)
Gapdh or TATA-Box Binding Protein (TBP) was utilized as a reference gene for qRT-PCR according to previous studies38,39,40,41,42.
Mouse embryonic fibroblasts
Mouse embryonic fibroblasts (MEFs) were obtained from wild type C57BL/6J mouse and Disc1-LI C57BL/6J mouse line. Embryos with embryonic day 14.5 were used. Body part of embryos without red blood organs was washed with PBS (Welgene, ML 008-01) and minced with blades. Samples were dissociated further with trypsin-EDTA (HyClone, SH30042.02) and pipetting for 10 min. Complete media (DMEM with 10% FBS and 1% penicillin/streptomycin) was applied to block trypsin activity. Collected cells were centrifuged for 3 min at 1000 rpm and pellets were resuspended with complete media. To dissociate into single cells, cell strainer was applied. Dissociated cells were seeded into culture dishes and media change was conducted after 6 h.
Circadian rhythm experiments
For cultured cell experiments, 1 μM of dexamethasone (Sigma, D4902-25MG) was applied to synchronize cells’ circadian phase. After 2 h, cells were changed to fresh complete media (DMEM with 10% FBS and 1% penicillin/streptomycin) and were harvested according to each time point. To obtain mouse brain samples for circadian experiments, mice were entrained to a 12-h light and 12-h dark cycle for 7 days and then to constant darkness to monitor internal circadian phenotypes. After the constant dark cycle had started, mice were kept for 1 day and sacrificed in the next day. 36 h after the constant dark cycle had started was considered circadian time 0. Mice were sacrificed under dim red light according to their circadian times and further dissection for brains was performed.
For long-term monitoring of circadian behavior in live animals, mice were surgically implanted with telemetric proves (E-mitter; Mini-Mitter, Bend) and kept in a light-proof Clean Animal Rack cabinet (Shin Biotech) equipped with an automatic light–dark schedule controller. The light intensity during the light phase was kept at 350–450 lux all over the cabinet. The temperature inside the cabinet was maintained to 23 ± 1 °C, and constant air ventilation was applied all the time. Prior to the actual monitoring, mice were fully entrained to a 12-h light and 12-h dark cycle for at least two weeks. For the next 10 days with the same light–dark cycle, wheel-running activity, body temperature, and home cage activity were continuously recorded at 6-min intervals using ER-4000 system. Data obtained during this period were used for the construction of light–dark profiles of behaviors. Then, mice were released to constant darkness (dark-dark cycle) for 10 days to determine the free-running period of circadian behaviors. For phase delay assay or phase advance assay, 30 min of light pulse was given to mice on circadian time 14 h or 20 h, respectively. Thereafter, the phase change was measured.
Luciferase assay
Luciferase assay was conducted using Dual Luciferase Assay system (Promega, E1910), according to manufacturer’s instruction. Cells were cultured on 12-well plate and transfected with PEI. Prior to cell prep, cells were washed with PBS (Welgene, ML 008-01). 100 μl of passive lysis buffer (PLB) was applied to each well, and the plate was agitated for 10 min to detach cells from the plate. The lysate was collected and centrifuged for 30 s at 13,000 rpm. The 40 μl of supernatant was transferred to 96-well white plate (Nunc, 136101). 50 μl of luciferase assay buffer II was injected into a well of 96-well plate, and shaking was performed for 1 second. Thereafter, Firefly luciferase activity was measured for 10 s. 50 μl of Stop & Glo Buffer was injected into the same well of 96-well plate, and shaking was performed for 1 s. Thereafter, Renilla luciferase activity was measured for 10 s. Injection of substrates and luminescence detection were programmed and conducted by automatic injector and plate reader (TECAN, infinite M200 PRO).
Immunoprecipitation and ubiquitination assay
Cultured cells, transfected with proper plasmids for each experiment, were lysed with Nonidet P-40 buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1% NP40, 5 mM EDTA, 1× protease inhibitor cocktail). Brain samples were homogenized in Nonidet P-40 buffer. Samples were sonicated and centrifuged at 13,000 rpm, 4 °C for 10 min. The supernatants were incubated with 500 ng of antibodies via rotation at 4 °C. Protein A beads (Roche, 05015979001) were washed three times with Nonidet P-40 buffer. Antibody incubated samples were added to washed beads and incubated further for 4 h at 4 °C with rotation. Beads, with bound proteins, were precipitated with 1 min of centrifugation at 2000 rpm, 4 °C. Beads were washed three times with Nonidet P-40 buffer and denatured with SDS sampling buffer at 95 °C for 10 min. Samples were further subjected to immunoblotting. For ubiquitination assay, cells had been transfected with plasmids, including HA-ubiquitin; thereafter, 10 mM of MG132 (Sigma, C2211-5MG) was treated for 5 h prior to the cell lysis. Further experimental procedure is identical to immunoprecipitation assay.
Chromatin immunoprecipitation
Cultured cells were transfected with appropriate plasmids. Cells were treated with 0.75% of formaldehyde (Sigma, 252549) dropwise and incubated for 10 min with slow agitation. 125 mM of glycine was treated to stop cross-linking. After twice of PBS washing, cells were harvested with PBS and centrifuged for 5 min, 1000 × g, 4 °C. Cells were resuspended with ChIP lysis buffer (50 mM HEPES-KOH pH 7.5, 140 mM NaCl, 1 mM EDTA pH 8.0, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 1× Protease Inhibitor) and incubated for 10 min on ice. Samples were sonicated for 20 min (30% amplitude, sonicate/rest for 3 s/3 s), and cell debris was removed with centrifuge for 10 min, 13,000 rpm, 4 °C. Samples were diluted with mRIPA buffer (150 mM NaCl, 50 mM Tris-HCl pH 7.5, 1% NP-40, 1% Triton X-100, 0.5% sodium deoxycholate, 5 mM EDTA). 50 μl of samples were kept as an input. 25 μg of total DNA samples were used each. Samples were incubated with Protein A beads (Roche, 05015979001) for 4 h at 4 °C with rotation to pre-clear non-specific bindings. Beads were removed by centrifuge for 1 min, 2,000 rpm, 4 °C. Antibody was treated to samples and incubated for 4 h at 4 °C with rotation. New Protein A beads were washed three times with mRIPA buffer, and beads were incubated with samples for overnight. Incubated beads were washed once with Low salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl pH 8.0, 150 mM NaCl), once with High salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl pH 8.0, 500 mM NaCl), once with LiCl buffer (0.25 M LiCl, 1% NP-40, 1% sodium deoxycholate, 1 mM EDTA, 10 mM Tris-HCl pH 8.0) by centrifuge for 1 min, 2000 rpm, 4 °C. Bead-attached samples were eluted from beads by incubation with 120 μl of Elution buffer (1% SDS, 100 mM NaHCO3) for 15 min at 30 °C. Beads were removed by centrifuge for 1 min, 2000 rpm. 4.8 μl of 5 M NaCl and 2 μl of 10 mg/ml RNase A (Thermo, EN0531) were added to samples and incubated for overnight at 65 °C with agitation. 4 μl of 10 mg/ml Proteinase K (Sigma, P2308) was added and incubated for 1 h at 60 °C with agitation. Further, DNA was acquired by PCR purification kit. For inputs, 50 μl of each input was mixed with 70 μl of Elution buffer and followed the same procedure with bead samples. Promoter targets were quantified with qPCR. For E-boxes of DISC1 promoter, 5′-GGTTTTGTGCCAAGCCTCTG-3′/5′-AGCTCACCTCCAGGCTAGAA-3′ and 5′-GGAGGTGAGCTGCTTAAGGG-3′/5’-CCATGCAAGCTCCTAGGCAA-3′ primer sets were utilized for qPCR.
Quantification and statistical analysis
Sample size was chosen based on previous studies. No statistical methods were used for sample size estimation. No randomization was used for animal studies. The investigators were not blinded to the group allocation during the animal experiments. ImageJ (NIH) was used for quantification of band intensity of western blot, and Prism 8 (GraphPad) was used for statistical analyses. Statistical significances were determined by unpaired t-test or paired t-test, two-tailed for comparisons of two groups. One-way ANOVA or two-way ANOVA was used for comparisons of multiple groups, followed by Tukey’s multiple comparison test or Sidak’s multiple comparison test, respectively. P-value less than 0.05 was considered a significant difference. If variances of the data were significantly different, we utilized a statistical test that does not assume homogenous variance; Welch’s ANOVA, unpaired t-test with Welch’s correction.
Results
DISC1 shows rhythmic expression relative to circadian time
To elucidate whether DISC1 is associated with the circadian system, we first examined whether DISC1 itself has an oscillatory expression pattern as other circadian-related genes. Disc1 mRNA expression was assessed in NIH3T3 cells after synchronization induced by dexamethasone treatment. The mRNA level of Disc1 showed an oscillatory pattern that peaked at 24 and 40 h after synchronization (Fig. 1a). Subsequently, we examined this expression pattern on an organism level. We entrained mice to a 12-h light and 12-h dark cycle for a week, and then switched to constant darkness and sacrificed mice according to their circadian time. Remarkably, we revealed the oscillatory mRNA expression of Disc1 in the hippocampus (Fig. 1b). Disc1 mRNA peaked at circadian time 20 h (CT20), originally nighttime (~12–24 h) for mice. The mRNA oscillation of Disc1 was also observed in the suprachiasmatic nucleus (SCN) (Fig. 1c). Disc1 mRNA peak was CT19. When compared to other circadian genes in SCN, Disc1 peak time is slower than Per1 and Per2, and faster than Rev-erbα43,44. Moreover, DISC1 endogenous protein levels also oscillated (Fig. 1d, e). Taken together, Disc1 expression shows an oscillatory pattern, with the expression level enhanced during late nighttime to early daytime.
a mRNA expression of Disc1 in NIH3T3 cells. Cells were treated with 1 μM of dexamethasone for 2 h to synchronize the circadian cycle. mRNA levels of Disc1 were analyzed by qRT-PCR and compared to those of Gapdh (n = 5 for 12 h, n = 6 for other time points, biological replicates). The ratios of the relative mRNA levels at each time point to the mRNA levels averaged across all time points were presented. p = 0.0151, one-way ANOVA. b mRNA level of Disc1 in hippocampus of wild type mice (n = 3, 3 mice for each time point) during the circadian time. Mice were entrained on a 12-h light/12-h dark cycle for a week; thereafter, mice were in the constant dark for a day and were sacrificed under dim red light according to their circadian time. mRNA levels of Disc1 were analyzed using qRT-PCR and compared to those of Gapdh. To compare the expression patterns of circadian genes measured by qRT-PCR each other, the ratios of the relative mRNA levels at each time point to the mRNA levels averaged across all time points were presented. p = 0.0037 for Disc1, one-way ANOVA. c mRNA levels of Disc1 in suprachiasmatic nucleus (SCN) of wild type mice (n = 3, 3 mice for 7 h; n = 4, 4 mice for other time points) during the circadian time. mRNA levels of Disc1 were analyzed by qRT-PCR and compared to those of TATA-box binding protein (TBP). The ratios of the relative mRNA levels at each time point to the mRNA levels averaged across all time points were presented. p = 0.0166, one-way ANOVA. d DISC1 protein levels according to the circadian time in hippocampus were assessed by western blot. Wild type mice were entrained on a 12-h light/12-hr dark cycle for a week; thereafter, mice were in the constant dark condition and sacrificed under dim red light according to their circadian time. Disc1 knockout (Disc1-LI mouse) sample was used as a negative control. Arrow indicates DISC1 bands. e Quantification of DISC1 protein level from (d) (n = 4 for each time point, biological replicates). Protein levels were quantified relative to tubulin. The ratios of the relative band intensity at each time point to the band intensities averaged across all time points were presented. p = 0.0042, Welch’s ANOVA. Data are means with SEM.
CLOCK and BMAL1 heterodimer regulates DISC1 promoter
Next, we attempted to discover the factors that contribute to the oscillatory expression of DISC1 within the molecular circadian system. As shown in Fig. 1b, the mRNA expression of Disc1 increases in late nighttime and a little later, roughly similar to the expression pattern of Cry1. Since the expression of circadian genes, such as Cry1, Cry2, and Per2, is controlled by CLOCK/BMAL1 heterodimer5, we hypothesized that Disc1 expression could be regulated with a comparable mechanism.
Based on the previous characterization of DISC1 promoter27, we further analyzed the region from −982 to +47 bp relative to the transcription start site (TSS). DISC1 promoter was cloned into pGL3 vector containing luciferase destabilized by PEST sequence28. We then assessed the activity of DISC1 promoter by luciferase assay system. Interestingly, DISC1 promoter activity was enhanced when CLOCK and BMAL1 were co-expressed, but not when CLOCK was expressed alone (Fig. 2a). Next, fragments of the DISC1 promoter were sub-cloned into pGL3 vector. The distal part of DISC1 promoter (-982 to -624 bp relative to TSS) was highly enhanced by CLOCK and BMAL1 co-expression (Fig. 2b). The middle part of DISC1 promoter (−623 to −324 bp relative to TSS) was also moderately enhanced by CLOCK and BMAL1 transfection, while the proximal part of DISC1 promoter (−323 to +47 bp relative to TSS) did not show significant enhancement. Therefore, the distal part of DISC1 promoter is the most responsive region to CLOCK/BMAL1.
a DISC1 promoter was cloned into pGL3 vector to monitor promoter activity by luciferase expression. Luciferase assay was performed in HEK293 cells overexpressing CLOCK or together with BMAL1 (n = 3, biological replicates). b Luciferase assay with DISC1 promoter sub-regions: distal part (−982 to −624 bp relative to TSS), middle part (−623 to −324 bp relative to TSS), and proximal part (−323 to +47 bp relative to TSS). Luciferase assay was performed in HEK293 cells overexpressing CLOCK and BMAL1 (n = 3, biological replicates). Each group was normalized to the respective control (empty vectors were transfected). c Luciferase assay with E-box-mutated DISC1 promoter. Luciferase assay was performed in HEK293 cells overexpressing CLOCK and BMAL1 (n = 3, biological replicates). Each group was normalized to the respective control (empty vectors were transfected). d Luciferase assay conducted using the distal part of DISC1 promoter with E-box mutations in HEK293 cells overexpressing CLOCK and BMAL1 (n = 3, biological replicates). Each group was normalized to the respective control (empty vectors were transfected). e Chromatin immunoprecipitation (ChIP) was performed in HEK293 cells transfected with CLOCK or BMAL1 or both to assess direct binding on E-box sequences of DISC1 promoter (n = 3, biological replicates). GFP antibody was utilized to pull down GFP-BMAL1. qPCR was used to quantify the E-box sequences precipitated by GFP-BMAL1. Red arrows indicate estimated primer binding sites for qPCR. For CLOCK and BMAL1 transfection, RFP-CLOCK-myc and GFP-BMAL1 constructs were used for (a–d), and (e). RLA represents relative luciferase activity. Data are means with SEM. *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001, one-way ANOVA and Tukey’s post-hoc test for (a–c), (d), and (e).
To define the binding sites of CLOCK/BMAL1 heterodimer on DISC1 promoter, we utilized the ‘LASAGNA-Search 2.0’ tool45,46. From the prediction based on the previously reported matrix parameter for CLOCK and BMAL147, two candidate binding sites (−718 and −668 bp relative to TSS) were identified (Supplementary Fig. 1). The two predicted sites match with the consensus E-box element, CANNTG, where CLOCK/BMAL1 binds48,49,50,51. Then, the predicted E-box sequences of DISC1 promoter were mutated to ‘ACNNTA’ to block their activity. DISC1 promoter with mutations in either one of the two E-box sequences or both showed lower responses to CLOCK and BMAL1 overexpression than the wild type promoter (Fig. 2c). When the distal part of DISC1 promoter was tested separately, double mutation of E-box sequences abrogated the response to CLOCK and BMAL1 (Fig. 2d). Then, chromatin immunoprecipitation assay (ChIP) was employed to test whether CLOCK/BMAL1 heterodimer physically binds to the E-box sequences of DISC1 promoter (Fig. 2e). In CLOCK/BMAL1-cotransfected cells, qPCR signals for both E-boxes (−718 and −668 bp relative to TSS) were markedly increased. In addition, we performed ChIP on mutant E-boxes (−718 and −668 bp relative to TSS) of DISC1 promoter to examine the binding of CLOCK/BMAL1 heterodimer. The qPCR signals for both E-boxes were significantly decreased (Supplementary Fig. 2). Altogether, the results further support the idea that the two E-box sites within the distal part of DISC1 promoter are critical for the modulation of DISC1 expression by CLOCK/BMAL1.
DISC1 regulates BMAL1 stability and expression of circadian genes
PERs and CRYs are regulated by CLOCK/BMAL1 through E-box sequences and exhibit oscillatory expression within circadian time5. Circadian expression of PERs and CRYs subsequently inhibits CLOCK/BMAL1 via a negative feedback loop. Since DISC1 expression is regulated by CLOCK/BMAL1 through E-box sequences and displays an oscillatory expression, we hypothesized that DISC1 protein may also affect the molecular circadian system, and we scrutinized the potential links. Interestingly, we observed that ectopic expression of DISC1 enhanced BMAL1 protein level in HEK293 cells (Fig. 3a, b). Conversely, endogenous BMAL1 protein level was decreased in mouse embryonic fibroblasts (MEFs) derived from Disc1-LI mice, a Disc1-deficient mouse line24 (Fig. 3c, d).
a GFP-BMAL1 and Flag-DISC1 were co-transfected into HEK293 cells. Empty Flag vector was used as a control. b Quantification of GFP-BMAL1 levels from (a) relative to tubulin control (n = 5, biological replicates). c Endogenous BMAL1 protein expression was decreased in Disc1 knockout (Disc1-LI) MEFs. d Quantification of BMAL1 protein levels from (c) with tubulin control (n = 5, biological replicates). e Endogenous BMAL1 protein levels were measured in MEFs following treatment with 1 μM of dexamethasone to synchronize the circadian cycle. f Quantification of BMAL1 protein levels from (e) relative to tubulin control (n = 3 for each time point, biological replicates). The ratios of the relative band intensity at each time point to the band intensities averaged across all time points were presented. g–j mRNA levels of circadian genes measured by qRT-PCR after dexamethasone synchronization (n = 3 for each time point, biological replicates). The expression of circadian genes was reduced in Disc1 knockout MEFs. The panels show levels of Per1 (g), Per2 (h), Cry1 (i), and Bmal1 (j). Data are means with SEM. *p ≤ 0.05, **p ≤ 0.01, #p ≤ 0.0001, unpaired t-test for (b, d); two-way ANOVA and Sidak’s multiple comparisons for (f–i), and (j).
To verify whether DISC1 deficiency affects BMAL1 oscillation over circadian time, we analyzed BMAL1 protein expression at each circadian time after dexamethasone treatment (Fig. 3e, f). The circadian oscillation of BMAL1 was significantly damped in Disc1-LI MEFs, and this effect was higher when BMAL1 levels were at their peak, namely 20 and 24 h after dexamethasone treatment. Moreover, the mRNA levels of circadian genes under the control of BMAL1 were concomitantly reduced in Disc1-LI MEFs (Fig. 3g–j). Given that the knockout of Disc1 damps BMAL1, thereby also decreasing the mRNA expression of Per2, Cry1, and Bmal1, these data strongly suggest that DISC1 may function as a stabilizer of BMAL1.
Disc1 knockout mice display altered circadian behavior
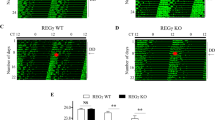
Since Disc1 knockout decreases the expression of circadian genes and decreases protein levels of BMAL1, we hypothesized that Disc1 knockout would also affect circadian behaviors. To test this hypothesis, we conducted circadian behavioral tests with Disc1-LI mice. We first entrained mice to a 12-h light and 12-h dark cycle prior to the observation of light/dark profile of circadian behavior. Notably, Disc1-LI mice exhibited lowered amplitude of circadian behaviors: wheel-running activity (Fig. 4a), body temperature (Fig. 4b), and home cage activity (Fig. 4c). We then monitored the wheel-running activity profile in a constant dark/dark cycle to observe the internal circadian period of mice, which is represented by the free-running period (Fig. 4d, e). Disc1-LI mice displayed a shorter free-running period than wild type mice. Furthermore, we examined phase perturbations from external light cues (Supplementary Fig. 3). We gave 30 min of the light pulse to mice at circadian time 14 h or 20 h for phase delay assay or phase advance assay, respectively. Disc1-LI mice exhibited a tendency toward an increase in phase delay and phase advance experiments. However, both did not reach statistical significance.
a–c Overall circadian behaviors during L/D cycle (n = 10, biological replicates). Mice were fully entrained to the light/dark (L/D; 12-h/12-h) cycle for at least two weeks. For the next 10 days with the same lighting schedule, light/dark profiles were generated as described in “Methods”. Then, mice were released to a dark/dark (D/D; constant dark) cycle for another 10 days to determine the internal circadian period. a WRA, wheel-running activity; b BT, body temperature; c HCA, home cage activity. Compared to wild type mice, Disc1 knockout mice (Disc1-LI) showed reduced values during the nighttime. Total measured values per one day were depicted as bar graphs for wheel-running and home cage activities. For body temperature, averaged values were used. d, e Free-running periods were measured for wild type (n = 16, biological replicates) and Disc1 knockout (n = 10, biological replicates) mice under the D/D cycle. Disc1 knockout mice showed a shorter Free-running period than wild type mice. f mRNA levels of circadian genes at circadian time of 4 h (CT04) and 16 h (CT16) (n = 4 for 4 h, n = 5 for 16 h, biological replicates). Hippocampus was dissected from wild type or Disc1 knockout mice. Data are means with SEM. *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001, unpaired t-test for (a), (b), (e) and (f); unpaired t-test with Welch’s correction for (c).
We also checked the expression of circadian genes at the transcript level. Consistent with the MEFs data, at circadian time 16 h, Disc1-LI mice showed lower expression of Per2, Cry1, and Bmal1 in the hippocampus than wild type mice (Fig. 4f). These results further demonstrate that DISC1 has a modulatory role for circadian rhythm likely through stabilizing BMAL1, a core component of the molecular clock.
DISC1 regulates the stability of BMAL1 by modulating its ubiquitination through GSK3β
Next, we sought to elucidate how DISC1 regulates the stability of BMAL1. Because DISC1 is known to function as a scaffold protein, we examined the interaction between DISC1 and BMAL1. DISC1 interacted with BMAL1 as shown by co-immunoprecipitation with overexpressed DISC1 and BMAL1 (Fig. 5a). We further confirmed the co-immunoprecipitation in MEFs and mouse brain lysates (Supplementary Fig. 4), suggesting that DISC1 may directly affect BMAL1 function.
a Co-immunoprecipitation of DISC1 and BMAL1. Flag-DISC1 and GFP-BMAL1 were transfected in HEK293 cells and precipitated with Flag-DISC1. b Ubiquitination assay for BMAL1. HA-ubiquitin was transfected in HEK293 cells to assess the ubiquitination of BMAL1. GFP-BMAL1 was transfected with or without Flag-DISC1. c Quantification of ubiquitin level from (b) relative to precipitated BMAL1 level (n = 5, biological replicates). d Ubiquitination assay of BMAL1 with DISC1 knockdown. HA-ubiquitin was transfected in HEK293 cells to assess the ubiquitination of BMAL1. myc-BMAL1 was transfected with shCTL or shDISC1 for knockdown of DISC1. e Quantification of ubiquitin level from (d) relative to precipitated BMAL1 level (n = 4, biological replicates). f Ubiquitination assay of BMAL1 with GSK3β S9A and DISC1. HA-ubiquitin was transfected in HEK293 cells to assess the ubiquitination of BMAL1. GFP-BMAL1 was transfected with myc-GSK3β S9A and Flag-DISC1 according to each combination of constructs. g Quantification of ubiquitin level from (f) relative to precipitated BMAL1 level (n = 4, biological replicates). h Western blot analysis of Y216 phosphorylation of GSK3β in wild type and Disc1 knockout (Disc1-LI) MEFs. MEFs were treated with 1 μM of dexamethasone to synchronize the circadian cycle. Western blot of GSK3β was conducted in different gel from others to avoid re-probing interference from previous band blot (p-Y216 GSK3β). Western blot was conducted in parallel with the same amount of identical samples. i Quantification of DISC1 and Y216 phosphorylation of GSK3β from (h) (n = 6, n = 3 for DISC1, biological replicates). Relative intensities of DISC1 and p-Y216 GSK3β relative to tubulin and total GSK3β, respectively, were subjected to analyses. The ratios of the relative band intensity at each time point to the band intensities averaged across all time points were presented. j Quantification of Y216 phosphorylation levels in wild type and Disc1 knockout MEFs from (h) (n = 6, biological replicates). The intensity of p-Y216 GSK3β relative to total GSK3β was subjected to analyses. The ratios of the relative band intensity at each time point to the band intensities averaged across all time points were presented. Data are means with SEM. *p ≤ 0.05, **p ≤ 0.01, paired t-test for (c, e), one-way ANOVA and Tukey’s post-hoc test for (g), two-way ANOVA and Sidak’s multiple comparisons for (j).
Ubiquitination is widely known as a signal for protein degradation52. Hence, we conducted ubiquitination assays and observed that ubiquitination of BMAL1 was inhibited upon co-expression of DISC1 (Fig. 5b, c), consistent with the finding that DISC1 increases BMAL1 levels. Conversely, ubiquitination of BMAL1 was enhanced in DISC1 knockdown cells (Fig. 5d, e). Thus, we concluded that DISC1 binds to BMAL1 and inhibits its ubiquitination.
The phosphorylation of glycogen synthase kinase 3β (GSK3β) Ser9 is inhibitory, while Tyr216 phosphorylation enhances its activity53. DISC1 can inhibit the Tyr216 phosphorylation (p-Y216) of GSK3β to impede its enzymatic activity30. On the other hand, GSK3β regulates multiple circadian proteins54, such as CRY255,56, CLOCK57, BMAL111, and REV-ERBα58, by modulating their stability10. Particularly, BMAL1 phosphorylation by GSK3β promotes its ubiquitination11. In addition, as shown in previous reports11,30, GSK3β interacts with DISC1 and BMAL1 in co-immunoprecipitation experiments (Supplementary Fig. 4b). Thus, we tested if DISC1 contributes to the regulation of BMAL1 ubiquitination through GSK3β. An activity-enhanced form of GSK3β by mutation of Ser9 to Ala increased the ubiquitination of BMAL1 as previously reported (Fig. 5f, g). However, this effect was abolished upon co-expression with DISC1.
To determine the effect of DISC1 on GSK3β over circadian time, we examined the level of p-Y216 in MEFs after dexamethasone synchronization (Fig. 5h). When DISC1 was at its peak, 24 h after synchronization, p-Y216 level was low (Fig. 5i). Subsequently, p-Y216 level gradually increased, while DISC1 level decreased. Moreover, p-Y216 level in Disc1-LI MEFs was increased at 28 h after dexamethasone treatment compared to wild type (Fig. 5j). Taken together, these results indicate that DISC1 suppresses p-Y216 of GSK3β according to circadian time, thereby enhancing BMAL1 stability.
Discussion
In this study, we showed that DISC1 has novel functions on the molecular circadian clock, further expanding the repertoire of the diverse roles of DISC1 in various cell types. Interestingly, the functional interaction between DISC1 and the molecular clock is reciprocal; DISC1 modulates the quality of the molecular clock by regulating the GSK3β-mediated BMAL1 stability and, at the same time, DISC1 expression itself is under control of CLOCK/BMAL1, displaying an oscillating expression pattern according to circadian time (Supplementary Fig. 5). The oscillating level of DISC1 is supposed to affect the oscillating level of BMAL1 protein, which will, in turn, affect the DISC1 promoter activity. This regulatory loop is achieved by the presence of E-box elements in the DISC1 promoter, which are targets of CLOCK/BMAL1 heterodimer. We identified the two major E-boxes controlled by BMAL1/CLOCK, but more cis-regulatory elements participating in the circadian expression of DISC1 directly or indirectly may still exist. This notion is supported by the observation that the DISC1 promoter activity was not totally abolished by the mutations of both E-box elements. Presumably, as a result, DISC1 deficiency leads to damped expressions of circadian genes. The impacts on the circadian genes were quantitatively differential (Fig. 3g–j), likely due to the heterogeneity of the compositions and their collective reactivity of the cis-regulatory elements responsible for circadian control59,60. Further clarification of this novel modulatory pathway will establish a complete view of the molecular clock.
The peak of DISC1 is similar to the peak of BMAL1, occurring ~20–24 h after dexamethasone treatment (Figs. 3f and 5i). Moreover, the expression pattern of DISC1 in the brain (Fig. 1b, c, e) is also consistent with the previously reported expression pattern of BMAL19,61,62, supporting a protective role of DISC1 for BMAL1. In this regard, the shorter period seen in the Disc1-LI mouse appears counterintuitive, given that mice with low BMAL1 show a more extended period63,64 and Bmal1 knockout mice exhibit arrhythmia65,66. Notably, however, DISC1 functionally down-regulates GSK3β by inhibiting p-Y216. Although there are conflicting results on the relationship between GSK3β and circadian period67,68, GSK3β gain-of-function tends to shorten the circadian period69,70. Thus, it is possible that the shortened period in Disc1-LI mice is due to the enhanced activity of GSK3β, which is consistent with the notion that DISC1 prevents BMAL1 from premature degradation to maintain the robustness of circadian rhythmicity.
We demonstrated that DISC1 expression oscillates according to circadian time. This finding suggests that the previously known functions of DISC1, from embryonic neurodevelopment to adult neurogenesis, may also be regulated by the circadian system. In this respect, the oscillating phosphorylation of Y216, a regulatory hotspot of GSK3β activity controlled by DISC130, is noteworthy. The phosphorylation of Y216 is critical for neural progenitor proliferation, which may indicate that DISC1-related neurodevelopmental processes, including neuronal signaling, neuronal morphogenesis, and spine regulation17, are also under circadian control. Moreover, DISC1 downregulation augments adult neurogenesis in the hippocampus71,72 in conjunction with memory formation73. Indeed, memory formation and adult neurogenesis are circadian-related phenomena74,75. For example, the number of BrdU-positive cells in the hippocampal subgranular zone changes over circadian time, with relatively impaired neurogenesis from late night to early daytime (peak neurogenesis at 15 h)75, which corresponds to the level of DISC1 protein expression in the hippocampus shown in this work (Fig. 1e). Therefore, it will be interesting to test whether DISC1 acts as an output pathway that links the molecular clock and neurodevelopmental program.
A natural deletion of Disc1, resulting in truncation after amino acid residue 528, did not alter the sleep phenotypes76, which is counterintuitive to our results from Disc1-LI mice. However, it is noteworthy that the truncated DISC1 still retains the region, amino acid residues 195-228, for the inhibition of GSK3β activity30, which is a crucial step of the regulation of BMAL1 stability by DISC1 according to the observations in this study. Its more robust circadian phenotype shown in this study is likely to be a reflection of complete Disc1 loss-of-function. Anyhow, the involvement of DISC1 in the regulation of the molecular circadian clock is intriguing in that sleep and circadian rhythm disruption (SCRD) is a common feature of various psychiatric disorders77. Components of molecular clocks were proposed as factors of SCRD78, though the precise molecular mechanisms underlying this phenomenon are still elusive. Although the genetic association of DISC1 with the various psychiatric conditions is still to be further clarified, the identification of DISC1 as a novel regulator of the molecular clock may suggest its role in the interface between some aspects of psychiatric disorders and the circadian system. Notably, rhythmic expression of genes, including PER2 and CRY2, is abolished in a group of patients with schizophrenia79; similarly, expression of PER2 and CRY1 is impaired in some schizophrenia patients80. In our study, an interesting correlation was observed in the Disc1 knockout mouse, where the expression of the circadian genes was also diminished. Thus, although the nature of the correlation needs further validation, the roles of DISC1 in the molecular clock may provide a novel mechanistic insight into the molecular links between SCRD and psychiatric disorders.
References
Young, M. W. & Kay, S. A. Time zones: a comparative genetics of circadian clocks. Nat. Rev. Genet. 2, 702–715 (2001).
Doherty, C. J. & Kay, S. A. Circadian control of global gene expression patterns. Annu. Rev. Genet. 44, 419–444 (2010).
Bell-Pedersen, D. et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat. Rev. Genet. 6, 544–556 (2005).
Dunlap, J. C. Molecular bases for circadian clocks. Cell 96, 271–290 (1999).
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017).
Gekakis, N. et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 (1998).
King, D. P. et al. Positional cloning of the mouse circadian clock gene. Cell 89, 641–653 (1997).
Kume, K. et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98, 193–205 (1999).
Shearman, L. P. et al. Interacting molecular loops in the mammalian circadian clock. Science 288, 1013–1019 (2000).
Hirano, A., Fu, Y. H. & Ptacek, L. J. The intricate dance of post-translational modifications in the rhythm of life. Nat. Struct. Mol. Biol. 23, 1053–1060 (2016).
Sahar S., Zocchi L., Kinoshita C., Borrelli E. & Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3 beta-mediated phosphorylation. PLoS ONE 5, e8561 (2010).
Busino, L. et al. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science 316, 900–904 (2007).
Godinho, S. I. H. et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science 316, 897–900 (2007).
Zhao, X. et al. Circadian amplitude regulation via FBXW7-targeted REV-ERBα degradation. Cell 165, 1644–1657 (2016).
Millar, J. K. et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum. Mol. Genet. 9, 1415–1423 (2000).
Niwa, M. et al. DISC1 a key molecular lead in psychiatry and neurodevelopment: no-more disrupted-in-schizophrenia 1. Mol. Psychiatry 21, 1488–1489 (2016).
Brandon, N. J. & Sawa, A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat. Rev. Neurosci. 12, 707 (2011).
Ishizuka, K., Paek, M., Kamiya, A. & Sawa, A. A review of disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol. Psychiatry 59, 1189–1197 (2006).
Wilkinson, B. et al. Endogenous cell type-specific DISC1 interactomes reveal protein networks associated to neurodevelopmental disorders. Biol. Psychiatry 85, 305–316 (2018).
Camargo, L. M. et al. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol. Psychiatry 12, 74 (2006).
Sawamura, N. et al. Nuclear DISC1 regulates CRE-mediated gene transcription and sleep homeostasis in the fruit fly. Mol. Psychiatry 13, 1138–1069 (2008).
Jaaro-Peled, H. et al. Abnormal wake/sleep pattern in a novel gain-of-function model of DISC1. Neurosci. Res. 112, 63–69 (2016).
Wulff, K., Porcheret, K., Cussans, E. & Foster, R. G. Sleep and circadian rhythm disturbances: multiple genes and multiple phenotypes. Curr. Opin. Genet. Dev. 19, 237–246 (2009).
Seshadri, S. et al. Interneuronal DISC1 regulates NRG1-ErbB4 signalling and excitatory–inhibitory synapse formation in the mature cortex. Nature. Communications 6, 10118 (2015).
Valenzuela, D. M. et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat. Biotechnol. 21, 652–659 (2003).
Longo, P. A., Kavran, J. M., Kim, M.-S. & Leahy, D. J. Transient mammalian cell transfection with polyethylenimine (PEI). Methods Enzymol. 529, 227–240 (2013).
Walker, R. M. et al. The DISC1 promoter: characterization and regulation by FOXP2. Hum. Mol. Genet. 21, 2862–2872 (2012).
Rechsteiner, M. & Rogers, S. W. PEST sequences and regulation by proteolysis. Trends Biochemical Sci. 21, 267–271 (1996).
Fukuda, T., Sugita, S. & Inatome, R. Yanagi S. CAMDI, a novel disrupted in schizophrenia 1 (DISC1)-binding protein, is required for radial migration. The. J. Biol. Chem. 285, 40554–40561 (2010).
Mao, Y. et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/β-catenin signaling. Cell 136, 1017–1031 (2009).
Kwak, Y. et al. Cyclin-dependent kinase 5 (Cdk5) regulates the function of CLOCK protein by direct phosphorylation. J. Biol. Chem. 288, 36878–36889 (2013).
Yoo, S.-H. et al. Period2 3′-UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc. Natl Acad. Sci. USA 2017, 06611 (2017).
Militi, S. et al. Early doors (Edo) mutant mouse reveals the importance of period 2 (PER2) PAS domain structure for circadian pacemaking. Proc. Natl Acad. Sci. USA 113, 2756–2761 (2016).
Yin, L., Joshi, S., Wu, N., Tong, X. & Lazar, M. A. E3 ligases Arf-bp1 and Pam mediate lithium-stimulated degradation of the circadian heme receptor Rev-erbα. Proc. Natl Acad. Sci. USA 107, 11614–11619 (2010).
Koike, H., Arguello, P. A., Kvajo, M., Karayiorgou, M. & Gogos, J. A. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc. Natl Acad. Sci. USA 103, 3693–3697 (2006).
Jang, H. et al. SREBP1c-CRY1 signalling represses hepatic glucose production by promoting FOXO1 degradation during refeeding. Nat. Commun. 7, 12180 (2016).
Lee, S. et al. Valproate alters dopamine signaling in association with induction of Par-4 protein expression. PLoS ONE 7, e45618–e45618 (2012).
Lin, S.-T. et al. Nuclear envelope protein MAN1 regulates clock through BMAL1. eLife 3, e02981 (2014).
Akagi, R. et al. Dysregulated circadian rhythm pathway in human osteoarthritis: NR1D1 and BMAL1 suppression alters TGF-β signaling in chondrocytes. Osteoarthr. Cartil. 25, 943–951 (2017).
Keller, M. et al. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl Acad. Sci. USA 106, 21407 (2009).
Hirano, A., Braas, D., Fu, Y.-H. & Ptáček, L. J. FAD Regulates CRYPTOCHROME protein stability and circadian clock in mice. Cell Rep. 19, 255–266 (2017).
Son, G. H. et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc. Natl Acad. Sci. USA 105, 20970 (2008).
DeBruyne, J. P. et al. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron 50, 465–477 (2006).
Ni, Y. et al. The involvement of sympathetic nervous system in essence of chicken-facilitated physiological adaption and circadian resetting. Life Sci. 201, 54–62 (2018).
Lee, C. & Huang, C.-H. LASAGNA-Search: an integrated web tool for transcription factor binding site search and visualization. Biotechniques 54, 141–153 (2013).
Lee, C. & Huang, C.-H. LASAGNA-Search 2.0: integrated transcription factor binding site search and visualization in a browser. Bioinformatics 30, 1923–1925 (2014).
Kielbasa, S. M., Gonze, D. & Herzel, H. Measuring similarities between transcription factor binding sites. BMC Bioinforma. 6, 237 (2005).
Oishi, K., Shirai, H. & Ishida, N. Identification of the circadian clock-regulated E-box element in the mouse plasminogen activator inhibitor-1 gene. J. Thromb. Haemost. 5, 428–431 (2007).
Ripperger, J. A. & Schibler, U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat. Genet. 38, 369–374 (2006).
Li, R. et al. CLOCK/BMAL1 regulates human nocturnin transcription through binding to the E-box of nocturnin promoter. Mol. Cell. Biochem. 317, 169–177 (2008).
Wang, Z., Wu, Y., Li, L. & Su, X. D. Intermolecular recognition revealed by the complex structure of human CLOCK-BMAL1 basic helix-loop-helix domains with E-box DNA. Cell Res. 23, 213–224 (2013).
Clague, M. J. & Urbé, S. Ubiquitin: same molecule, different degradation pathways. Cell 143, 682–685 (2010).
Beurel, E., Grieco, S. F. & Jope, R. S. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol. Ther. 148, 114–131 (2015).
Reischl, S. & Kramer, A. Kinases and phosphatases in the mammalian circadian clock. FEBS Lett. 585, 1393–1399 (2011).
Kurabayashi, N., Hirota, T., Sakai, M., Sanada, K. & Fukada, Y. DYRK1A and glycogen synthase kinase 3β, a dual-kinase mechanism directing proteasomal degradation of CRY2 for circadian timekeeping. Mol. Cell. Biol. 30, 1757–1768 (2010).
Harada, Y., Sakai, M., Kurabayashi, N., Hirota, T. & Fukada, Y. Ser-557-phosphorylated mCRY2 Is degraded upon synergistic phosphorylation by glycogen synthase kinase-3β. J. Biol. Chem. 280, 31714–31721 (2005).
Spengler, M. L., Kuropatwinski, K. K., Schumer, M. & Antoch, M. A serine cluster mediates BMAL1-dependent CLOCK phosphorylation and degradation. Cell Cycle 8, 4138–4146 (2009).
Yin, L., Wang, J., Klein, P. S. & Lazar, M. A. Nuclear receptor rev-erbα is a critical lithium-sensitive component of the circadian clock. Science 311, 1002–1005 (2006).
Yamajuku, D. et al. Identification of functional clock-controlled elements involved in differential timing of Per1 and Per2 transcription. Nucleic Acids Res. 38, 7964–7973 (2010).
Ukai-Tadenuma, M. et al. Delay in feedback repression by cryptochrome 1 is required for circadian clock function. Cell 144, 268–281 (2011).
Chen, L. et al. BMAL1 Disrupted intrinsic diurnal oscillation in rat cerebrovascular contractility of simulated microgravity rats by altering circadian regulation of miR-103/CaV1.2 signal pathway. Int. J. Mol. Sci. 20, 3947 (2019).
Barca-Mayo, O. et al. Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat. Commun.s 8, 14336 (2017).
Landgraf, D. et al. Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biol. Psychiatry 80, 827–835 (2016).
Mieda, M. et al. Cellular clocks in AVP neurons of the SCN are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron 85, 1103–1116 (2015).
Bunger, M. K. et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000).
McDearmon, E. L. et al. Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science 314, 1304–1308 (2006).
Hirota, T. et al. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3β. Proc. Natl Acad. Sci. USA 105, 20746–20751 (2008).
Besing, R. C. et al. GSK3 activity regulates rhythms in hippocampal clock gene expression and synaptic plasticity. Hippocampus 27, 890–898 (2017).
Iitaka, C., Miyazaki, K., Akaike, T. & Ishida, N. A role for glycogen synthase kinase-3β in the mammalian circadian clock. J. Biol. Chem. 280, 29397–29402 (2005).
Martinek, S., Inonog, S., Manoukian, A. S. & Young, M. W. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105, 769–779 (2001).
Terrillion, C. E. et al. DISC1 in astrocytes influences adult neurogenesis and hippocampus-dependent behaviors in mice. Neuropsychopharmacology 42, 2242–2251 (2017).
Duan, X. et al. Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130, 1146–1158 (2007).
Kempermann G., Song H. J. & Gage F. H. Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol. 7, a018812 (2015).
Gerstner, J. R. & Yin, J. C. P. Circadian rhythms and memory formation. Nat. Rev. Neurosci. 11, 577–588 (2010).
Bouchard-Cannon, P., Mendoza-Viveros, L., Yuen, A., Kærn, M. & Cheng, H.-Y.M. The circadian molecular clock regulates adult hippocampal neurogenesis by controlling the timing of cell-cycle entry and exit. Cell Rep. 5, 961–973 (2013).
Dittrich, L., Petese, A. & Jackson, W. S. The natural Disc1-deletion present in several inbred mouse strains does not affect sleep. Sci. Rep. 7, 5665 (2017).
Wulff, K., Gatti, S., Wettstein, J. G. & Foster, R. G. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 11, 589–599 (2010).
Jagannath, A., Taylor, L., Wakaf, Z., Vasudevan, S. R. & Foster, R. G. The genetics of circadian rhythms, sleep and health. Hum. Mol. Genet. 26, R128–R138 (2017).
Seney, M. L. et al. Diurnal rhythms in gene expression in the prefrontal cortex in schizophrenia. Nat. Commun. 10, 3355 (2019).
Johansson, A.-S., Owe-Larsson, B., Hetta, J. & Lundkvist, G. B. Altered circadian clock gene expression in patients with schizophrenia. Schizophr. Res. 174, 17–23 (2016).
Acknowledgements
This work was supported by the Advanced Research Center Program (Organelle Network Research Center, 2017R1A5A1015366), the Brain Research Program (2017M3C7A1047875) and the Bio & Medical Technology Development Program (NRF-2020M3E5E2039894) funded by Korean National Research Foundation (S.K.P.).
Funding
This research was also supported by KBRI basic research program funded by Ministry of Science and ICT (21-BR-03-01).
Author information
Authors and Affiliations
Contributions
S.B.L., Y.K., Y.-U.P., and S.K.P. conceived the study. S.B.L., S.C., and S.K.P. designed experiments. S.B.L., J.P., Y.K., Y.-U.P., T.T.M.N., B.K.S., Y.W., and E.C. performed experiments. S.B.L., B.K.S., Y.W., Y.S., S.C., and S.K.P. analyzed and interpreted data. S.B.L., S.C., and S.K.P. wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, S.B., Park, J., Kwak, Y. et al. Disrupted-in-schizophrenia 1 enhances the quality of circadian rhythm by stabilizing BMAL1. Transl Psychiatry 11, 110 (2021). https://doi.org/10.1038/s41398-021-01212-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-021-01212-1