Abstract
Introduction
Patients with complete spinal cord injury (SCI) may develop concurrent sequalae that interact and share symptoms; thus, a careful approach to diagnosis and management of new symptoms is crucial.
Case presentation
A patient with prior T4 complete SCI presented with progressive autonomic nervous system (ANS) dysfunction. The initial differential diagnosis included syringomyelia and lumbar Charcot arthropathy. He had comorbid heterotopic ossification (HO) of the left hip. Surprisingly, his autonomic symptoms resolved following resection of the HO. In hindsight, loss of motion through the hip caused by HO may have led to hinging through a previously asymptomatic lumbar Charcot joint, causing dysautonomia.
Discussion
ANS dysfunction is a disabling sequela of complete SCI and has a broad differential diagnosis. Hip immobility may be an indirect and overlooked cause due to the mechanical relationship between the hip and the lumbar spine.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) can result in numerous secondary conditions that co-exist, interact, and share symptoms. These include post-traumatic syringomyelia, spinal cord tethering, chronic urinary tract infections (UTI), osteodiscitis-osteomyelitis, and multiple arthropathies including heterotopic ossification (HO) and Charcot spine.
Jean-Martin Charcot first described the correlation between loss of sensation in a joint and subsequent arthropathy in 1868 in patients with tabes dorsalis [1]. Loss of proprioceptive feedback from a weight-bearing joint, combined with loss of normal movement about it, can lead to joint degeneration, fracture, and bone resorption. This leads in turn to joint deformity and instability. Charcot arthropathy may remain symptomatically silent until severe instability activates pain signaling pathways, usually in response to movement. Charcot spine may remain completely asymptomatic for many years. In some cases, however, it may become associated with autonomic dysreflexia and patients may display symptoms including paroxysmal hypertension, reflex bradycardia, headache, sweating disturbances, and skin flushing [2,3,4,5].
Here we present a case of autonomic nervous system (ANS) dysfunction associated with the sitting position in the setting of Charcot arthropathy of the lumbosacral spine, left hip HO, and progressive syringomyelia. The patient’s autonomic symptoms completely resolved following resection of heterotopic bone that restored motion of the left hip joint. This is the first report in the literature describing resolution of ANS dysfunction following resection of hip HO.
Case presentation
History and physical examination
The patient is a 59-year-old man. He sustained a T4 AIS A complete SCI in a motorcycle accident 40 years ago. For decades, experienced mild, intermittent, non-positional bilateral thigh sweats, accompanied by feeling cold. He was admitted to the SCI rehabilitation unit for treatment of complicated urosepsis. During and following resolution of the urosepsis, his autonomic symptoms became much more severe than baseline, and newly associated with changes in position. The symptoms included thigh sweating, feeling cold with chills and shivering, fever as high as 38.3–39.4° C, abdominal and thigh spasms, diaphoresis, and tachycardia with heart rate in the 120–140 s. Systolic blood pressure remained low 90–110 s during these episodes, which prohibited diagnosis with autonomic dysreflexia [6]. These ANS-related symptoms consistently occurred following being assisted from lying flat to sitting. On examination, he was noted to have full strength in bilateral upper extremities, with the exception of 4+/5 in the left triceps, and 0/5 strength in bilateral lower extremities where he had spasticity and increased tone. He had a T4 sensory level, above which he had intact light touch, pain, and temperature sensation.
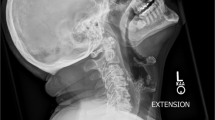
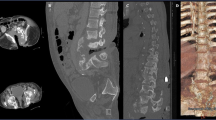
The initial workup and treatment discussion focused on the spine. Imaging studies revealed a lumbosacral Charcot joint (Fig. 1) and enlargement of a chronic post-traumatic cervicothoracic syrinx (Fig. 2). When positional ANS dysfunction persisted following resolution of the UTI, the Charcot spine and the cervicothoracic syrinx were the top two differential diagnoses. To assess lumbar spinal motion, flexion–extension lumbar radiographs were completed, but the images were not informative due to patient habitus (Body Mass Index 36 kg/m2) and positioning restrictions. Lumbosacral osteomyelitis was ruled out. To assess the enlarged cervicothoracic syrinx, a CT myelogram with lumbar dye injection was attempted to visualize subarachnoid adhesions and spinal cord tethering that could represent surgical targets for treatment of the syringomyelia. This was aborted because the lumbar cistern was technically inaccessible. A cisterna magna tap for dye injection was considered.
Compared with a normal mid-sagittal CT of the lumbosacral spine (a), mid-sagittal CT of the lumbosacral spine in this patient (b) reveals widespread destruction of the normal vertebral anatomy. The patient’s image (b) shows bone resorption, bony hypertrophy, and loss of structural stability from the fourth lumbar vertebra through the sacrum. Sagittal T2 MRI (c) and axial T2 MRI (d) at the level identified (dashed line, c) show a massive soft tissue callous (dashed outline, d) that has formed in response.
At the same time, the patient was coping with progressive difficulty sitting in his wheelchair. This had developed over the course of 1 year. Workup shortly before referral to orthopedic surgery included X rays that revealed a large mass of heterotopic bone that nearly bridged across the left hip (Fig. 3a). Physical examination revealed a mass of bone palpable immediately under the skin overlying the left hip and evidence of healed ulcers. The patient’s hip was fixed in 15° of flexion and in external rotation. On the right side, he had a dislocated hip, and on exam showed 10–35° of flexion. The patient was offered resection of left hip HO given significant morbidity of inability to flex the hip and failure of conservative measures. Further spinal workup was deferred.
Surgical technique
The patient had surgery to remove the hip HO 15 days after he was initially seen by the orthopedic surgery service. Under general endotracheal anesthesia, the patient was positioned in the lateral decubitus position. The degree of HO made identification of normal anatomy difficult. The shaft of the femur was palpated and traced up to the greater trochanter. A typical posterolateral approach was used. The iliotibial band was split. Immediately deep to that, an enormous mass of heterotopic bone was encountered. Dissection on the surface of the bone revealed the extent of the heterotopic bone. Osteotomes and rongeurs were used to remove the heterotopic bone. Bleeding was controlled, and the wound was irrigated. A drain was placed. The iliotibial band, subcutaneous tissues, and skin were closed. Immediately following the operation, we were able to move the patient’s left hip beyond neutral rotation and into internal rotation, and to flex the left hip 75–80°.
Postoperative course
Postoperatively, we prohibited range of motion of the hip until the incision had healed. The patient was treated with Indomethacin and Etidronate for HO prophylaxis. He sat 6 days postoperatively with normal vital signs and no autonomic symptoms. He was transferred to the SCI rehabilitation unit. His left hip range of motion continued to improve postoperatively: 45° at 4 weeks, 75° at 6 weeks, 85° at 7 weeks, and 90° at 8 weeks. His rehab hospitalization was prolonged due to UTI, hiatal hernia, and multiple gluteal abscesses requiring IR-guided drainage and antibiotics; through all of these complications, notably including the UTI, the ANS dysfunction did not recur. Four months, 7 months, and 1 year postoperatively, the patient reported continued resolution of all autonomic symptoms, and this was confirmed with his physical therapy sessions. On telephone follow-up at 4 years, the patient continued to deny any recurrence of his autonomic symptoms, and to decline further spinal imaging. 6-month and 2-year postoperative hip radiographs did not show HO recurrence (Fig. 3b, c).
Discussion
In a patient with complete SCI, painful stimuli below the level of the injury may result in autonomic symptoms. Charcot spine is suspected in patients with chronic complete SCI and new autonomic symptoms. The indications for instrumented fusion for the treatment of Charcot spine include severe spinal deformity, sitting imbalance, spinal instability due to fracture and/or vertebral body destruction, autonomic dysreflexia, progressive neurologic symptoms, and osteomyelitis [7,8,9,10]. The preferred approach is a combined anterior–posterior instrumented fusion [11]. The surgery is extensive and the rehabilitation long. Perioperative and long-term complications are common: 29% of patients have hardware complications requiring revision surgery [11, 12], and the incidence of deep infections is high [13].
In this report we describe a patient with T4 AIS A complete SCI, a significant lumbosacral Charcot joint, an enlarging chronic syrinx from the obex to T4, and hip HO. He developed very disabling autonomic symptoms associated with changes in position. Multiple interventions were contemplated to address these symptoms, including treatment of syringomyelia and surgical fusion of the Charcot joint. The patient deferred workup of the syringomyelia in favor of surgical treatment of left hip HO. This surgery restored a substantial unrestricted passive range of motion to the affected hip. To our initial surprise, the autonomic symptoms subsided following the hip procedure. The patient remains without disabling autonomic symptoms 4 years after the surgery.
This case represents an unusual variant of hip-spine syndrome. The patient’s autonomic symptoms appear to be caused by the lumbosacral Charcot, and yet they surprisingly resolved following HO resection. It is unclear what exactly caused the Charcot spine in this patient. While it can occur in a chronic fashion in patients with AIS A, it is also possible that the patient sustained damage to the lumbar spine and left hip in the initial injury decades ago and this continued to progress slowly. The latter is supported by the fact that the patient complained of mild autonomic symptoms for decades. The hip and spine pathology interact here in three important ways: (1) because of hip immobility secondary to HO, attempts to seat the patient may have resulted in hinging through the lumbosacral Charcot, triggering autonomic symptoms, (2) resection of the HO may have led to resolution of the autonomic symptoms by converting a symptomatic Charcot spine back to an asymptomatic Charcot spine, and (3) fusion of the Charcot spine prior to or in the absence of treatment of the HO would likely have failed, given the patient’s hip fixed at 15° of flexion.
Our case report suggests that hip pathology should be considered in the differential diagnosis for new or worsening autonomic dysfunction in patients with complete SCI. In some cases, performing the less complex and potentially morbid operation may obviate the indication for the more complex and potentially morbid operation. It is important to note, however, that this patient continues to have a large Charcot spine deformity and cervicothoracic syrinx. He may need to undergo treatment for one or both of these pathologies in the future. The extent of the syrinx specifically raises concern regarding the left triceps weakness, and he will need to be monitored closely for progression of neurologic symptoms.
HO recurs. The prophylactic use of NSAIDs here reflects surgeon preference. NSAIDs and postoperative low dose radiation are equally efficacious in preventing HO recurrence [14, 15].
There are two reported cases in which AD was associated with hip disease and resolved after corrective surgery. One patient had severe bilateral hip osteoarthritis and autonomic symptoms that developed decades after the index SCI. His symptoms resolved following bilateral hip arthroplasty [16]. Spinal imaging was notable for severe lumbar spine osteophytes causing ankylosis. A second patient with SCI developed AD after a fall from his wheelchair resulting in a pelvic fracture [17]. His symptoms resolved after the fracture healed 3 months later. Unfortunately, spinal imaging was not included in their workup.
This case demonstrates the complexity of caring for patients with longstanding SCI. In evaluating these patients, one has to be aware of the multifactorial origin of symptoms. This case highlights the importance of considering the hip joint in SCI patients with worsening autonomic symptoms.
References
Hoche G, Sanders LJ. On some arthropathies apparently related to a lesion of the brain or spinal cord, by Dr. J.-M. Charcot, January 1868. J Hist Neurosci. 1992;1:75–87.
Wirth CR, Jacobs RL, Rolander SD. Neuropathic spinal arthropathy. A review of the Charcot spine. Spine. 1980;5:558–67.
Gibson JL, Vuong SM, Bohinski RJ. Management of autonomic dysreflexia associated with Charcot spinal arthropathy in a patient with complete spinal cord injury: case report and review of the literature. Surg Neurol Int. 2018;9:113.
Ledbetter LN, Salzman KL, Sanders RK, Shah LM. Spinal neuroarthropathy: pathophysiology, clinical and imaging features, and differential diagnosis. Radiographics. 2016;36:783–99.
Karlsson AK. Autonomic dysfunction in spinal cord injury: clinical presentation of symptoms and signs. Prog Brain Res. 2006;152:1–8.
Krassioukov AV, Karlsson AK, Wecht JM, Wuermser LA, Mathias CJ, Marino RJ. Assessment of autonomic dysfunction following spinal cord injury: rationale for additions to International Standards for Neurological Assessment. J Rehabil Res Dev. 2007;44:103–12.
Aebli N, Potzel T, Krebs J. Characteristics and surgical management of neuropathic (Charcot) spinal arthropathy after spinal cord injury. Spine J. 2014;14:884–91.
Selmi F, Frankel HL, Kumaraguru AP, Apostopoulos V. Charcot joint of the spine, a cause of autonomic dysreflexia in spinal cord injured patients. Spinal Cord. 2002;40:481–3.
Moreau S, Lonjon G, Jameson R, Judet T, Garreau de Loubresse C. Do all Charcot spine require surgery? Orthop Traumatol Surg Res. 2014;100:779–84.
Solinsky R, Donovan JM, Kirshblum SC. Charcot Spine following chronic spinal cord injury: an analysis of 201 published cases. Spinal Cord. 2019;57:85–90.
Lee D, Dahdaleh NS. Charcot spinal arthropathy. J Craniovertebral Junction Spine. 2018;9:9–19.
Brown CW, Jones B, Donaldson DH, Akmakjian J, Brugman JL. Neuropathic (Charcot) arthropathy of the spine after traumatic spinal paraplegia. Spine. 1992;17 Suppl 6:S103–8.
Hong J, Sanfilippo JA, Rihn J, Fernandez C, Winegar CD, Friel B, et al. Complications in the management of Charcot spinal arthropathy. J Neurosurg Spine. 2009;11:365–8.
Vavken P, Castellani L, Sculco TP. Prophylaxis of heterotopic ossification of the hip: systematic review and meta-analysis. Clin Orthop Relat Res. 2009;467:3283–9.
Cai L, Wang Z, Luo X, She W, Zhang H. Optimal strategies for the prevention of heterotopic ossification after total hip arthroplasty: a network meta-analysis. Int J Surg. 2019;62:74–85.
Bickelhaupt B, Richard M, Trbovich M. Advanced hip osteoarthritis causing autonomic dysreflexia and severe spasticity in a patient with spinal cord injury: a case report. PMR. 2017;9:1047–50.
Kriz J, Andel R, Hakova R. Delayed diagnosis of an unsuspected pelvic fracture in a patient with tetraplegia. J Spinal Cord Med. 2014;37:425–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fatemi, P., Prolo, L.M., Giori, N.J. et al. Resection of hip heterotrophic ossification leads to resolution of autonomic nervous system dysfunction in a patient with spinal Charcot arthropathy: a case report. Spinal Cord Ser Cases 6, 41 (2020). https://doi.org/10.1038/s41394-020-0286-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-020-0286-5