Abstract
Study design
Cross-sectional study.
Objectives
This study: (1) investigated the accuracy of bioelectrical impedance analysis (BIA) and skinfold thickness relative to dual-energy X-ray absorptiometry (DXA) in the assessment of body composition in people with spinal cord injury (SCI), and whether sex and lesion characteristics affect the accuracy, (2) developed new prediction equations to estimate fat free mass (FFM) and percentage fat mass (FM%) in a general SCI population using BIA and skinfolds outcomes.
Setting
University, the Netherlands.
Methods
Fifty participants with SCI (19 females; median time since injury: 15 years) were tested by DXA, single-frequency BIA (SF-BIA), segmental multi-frequency BIA (segmental MF-BIA), and anthropometry (height, body mass, calf circumference, and skinfold thickness) during a visit. Personal and lesion characteristics were registered.
Results
Compared to DXA, SF-BIA showed the smallest mean difference in estimating FM%, but with large limits of agreement (mean difference = −2.2%; limits of agreement: −12.8 to 8.3%). BIA and skinfold thickness tended to show a better estimation of FM% in females, participants with tetraplegia, or with motor incomplete injury. New equations for predicting FFM and FM% were developed with good explained variances (FFM: R2 = 0.94; FM%: R2 = 0.66).
Conclusions
None of the measurement techniques accurately estimated FM% because of the wide individual variation and, therefore, should be used with caution. The accuracy of the techniques differed in different subgroups. The newly developed equations for predicting FFM and FM% should be cross-validated in future studies.
Similar content being viewed by others
Introduction
A spinal cord injury (SCI) often leads to paresis, which can cause muscle atrophy, metabolic disorder, and physical inactivity [1]. The physiological and lifestyle changes result in a decrease of fat free mass (FFM) and an increase in fat mass (FM), leading to a higher prevalence of obesity and several adverse metabolic sequelae such as insulin resistance, hyperlipidemia, and cardiovascular diseases in people with chronic SCI [2]. The loss of FFM is mainly due to the reduction in muscle mass. This reduction could reach up to over 50% in the first 6 months after injury in unloaded muscles [3]. To monitor their health status, identifying individuals at risk with percentage fat mass (FM%) ≥25% [4] and intervening with dietary advice and exercise prescription when considered necessary, regular assessment of body composition with accurate methods in people with SCI is essential.
Several techniques have been used to assess body composition in people with SCI, with dual-energy X-ray absorptiometry (DXA) considered as the gold standard [5]. However, DXA can have limited accessibility for many practitioners because of financial and logistical restrictions [6]. Therefore, feasible and relatively accurate alternative methods are needed for rehabilitation centers or dieticians. One of such methods that shows a good estimation of FM% in the general population is skinfold thickness [7]. The equation by Durnin and Womersley based on the values of four skinfold sites has been widely used in both the general population and people with SCI [6, 8,9,10]. Recently, Goosey-Tolfrey et al. developed a new prediction equation that well-predicted FM% (R2 = 0.84) in wheelchair athletes [10]; however, it has not been validated in the general population with SCI. Bioelectrical impedance analysis (BIA), another body composition measurement, has been extensively used because of its rapid processing, noninvasiveness and relative inexpensiveness [11]. Specifically, single-frequency BIA (SF-BIA) and segmental multi-frequency BIA (segmental MF-BIA) are widely used in both healthy people and patients by using the measured impedance of electric current to determine body water and calculate FFM and FM. Buchholz et al. suggested that after adding some relevant parameters including age, sex, height, and body mass to BIA results, body composition can be well-predicted by using SF-BIA in people with paraplegia [12]. However, prediction equations are population-specific, therefore the accuracy of BIA and skinfold thickness may vary with sex and SCI group (e.g., tetraplegia vs. paraplegia) [13]. Until now, studies about the accuracy of BIA and skinfold thickness to evaluate body composition in people with SCI are limited and most of them have focused on athletes instead of the general population with SCI [6, 9, 10, 14, 15]. These studies have shown that BIA and skinfolds systematically underestimated FM% (BIA: 5.1–10.7%; skinfolds: 5.8–15.2%) compared to DXA. Spungen et al. found that DXA, BIA, and skinfolds are equally valuable in estimating body fat in a general population with SCI [15]. However, they only included 12 people with tetraplegia. Furthermore, equations to estimate body composition have been created only for wheelchair athletes with SCI [10]. This population tends to have lower FM% compared to the general population with SCI because of the high training loads [10]. Therefore, the aim of this study was twofold: (1) to investigate the accuracy of BIA and skinfold thickness relative to DXA in the assessment of body composition in a general population with SCI and whether sex and lesion characteristics affect the accuracy, and (2) to develop new prediction equations to estimate FFM and FM% in a general population with SCI using BIA and skinfolds outcomes.
Methods
Participants
Fifty community-dwelling men and women with SCI, including two wheelchair tennis athletes and two wheelchair rugby athletes, were recruited via a rehabilitation center and the Dutch SCI patient organization. Inclusion criteria were: people with SCI (not ventilator-dependent), aged 18–75 years, time since injury ≥12 months. Exclusion criteria were: artificial pacemaker, epilepsy, pregnancy, fever (>38.5 degrees), progressive illness, psychiatric disorders, severe communication obstacles, pressure ulcers, or abnormal hydration status due to e.g., edema. This study was approved by the Medical Ethical Committee of Slotervaart hospital and Reade (NL64704.048.18). All participants signed an informed consent form before data collection.
Design
During a visit, the measurements of body composition were conducted. All measurements were scheduled with participants in a fasting state. Caffeine, alcohol intake and heavy physical exercise were not allowed <24 h before the measurements. Measurement order was always DXA, SF-BIA, segmental MF-BIA, and skinfold thickness.
Personal and lesion characteristics
Age, sex, lesion level, and completeness were collected by asking participants. Lesion characteristics were determined using the AIS criteria [16]. Lesion level was defined by tetraplegia (lesion ≥T1) and paraplegia (<T1) while motor complete injury was defined by AIS A or B and motor incomplete injury by AIS C or D.
Dual-energy X-ray absorptiometry
Whole-body and segmental FFM (lean mass plus bone mineral content), FM and FM% were assessed using DXA (Hologic Discovery, Hologic Inc., Waltham, Mass, USA). Standard quality control (local Standard Operating Procedure) was performed with the calibration of the lumbar spine phantom before every measurement on a daily basis and the weekly calibration of radiographic uniformity. Participants were instructed to wear loose fitting clothes and remove all jewelry. All scans and analyses were performed by the same trained operator. To minimize produced spasms and any discomfort on the DXA table, participants were positioned as closely as possible to the standard protocol, with a strap used to ensure there was no leg movement during the scan.
Bioelectrical impedance analysis
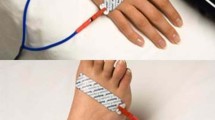
Bioelectrical impedance was measured using two methods. The Bodystat 1500MDD (Bodystat® 1500MDD, Bodystat Inc, Douglas, United Kingdom) determined whole-body composition by the measurement of resistance and reactance at 50 kHz (SF-BIA). The InBody S10 determined (InBody® S10, InBody Bldg., Seoul, South-Korea) whole-body and segmental body composition by the measurements of resistance and reactance at multiple frequencies (1, 5, 50, 250, 500 and 1000 kHz; segmental MF-BIA). Based on the BIA principle, the impedance of a cylindrical conductor is related to its length. This indicates that the impedance index height2 (cm)/impedance (h2/Z; with Z the impedance given by BIA) has a stronger linear relationship with total body water (TBW) and FFM than impedance alone [17]. Thus, h2/Z by BIA can be a significant predictor of body composition. Participants were instructed to lie down in a supine position with arms and legs slightly apart on a nonconductive surface for 5–10 min before the measurement started. According to the standard operating procedures, four adhesive electrodes were used for measurement with the SF-BIA on the right side of the body while six touching electrodes (three on each side) were used for measurement with the segmental MF-BIA on both sides of the body (Fig. 1).
Anthropometry
Height was measured to the nearest 0.1 cm in a supine position with a measuring tape. For the participants who were unable to lie fully straight, the measured values were compared with the self-reported height. If the values differed, an extra measurement was performed to confirm the final measured value.
Body mass was measured to the nearest 0.1 kg with a wheelchair accessible weighing scale (BL-5R, Christen Swiss B.V., Geldermalsen, the Netherlands). The mass of the wheelchair and clothes were deducted from the total mass afterwards.
Waist circumference was measured three times to the nearest 0.1 cm in a supine position with a measuring tape at the umbilicus level after a normal expiration. Calf circumference was measured three times with a measuring tape at the maximum girth of the right calf. The median value was taken for analysis according to the International Standards for Anthropometric Assessment (ISAA) [18].
Eight skinfold sites (biceps, triceps, subscapular, iliac crest, supraspinal, abdominal, front thigh and medial calf) were marked with a dermatographic pen and measured following the guidelines by ISAA using a Slim Guide Skinfold Caliper (Creative Health Products, Michigan, USA) by the same trained operator [18]. Measurement at each site was made in triplicate with the participant in an upright sitting position in his/her wheelchair. The median value was taken for analysis. In case a measured value varied more than 2 mm compared to the previous measurement, an extra measurement was taken to replace that value. The equation by Durnin–Womersley (established for able-bodied people: Body density = c–m × log∑SKF4, measured sites: biceps, triceps, subscapular and iliac crest, c and m are given values varying with age and sex) was used to estimate body density [8]. Conversion of body density to FM% was calculated using the Siri equation [19]. The equation by Goosey-Tolfrey et al. (established for wheelchair athletes: FM% = −3.04 + 0.41 × ∑SF7 − 0.001 × ∑SF72 + 0.03 × Calf circumference, measured sites: biceps, triceps, subscapular, supraspinal, abdominal, front thigh, and medial calf and calf circumference) was used to estimate FM% [10].
Statistical analysis
The normality of the data was checked by the Shapiro–Wilk test and Q–Q plots and all data were normally distributed. Paired sample t tests were used to determine the systematic difference in measuring body composition between DXA, the BIA methods and skinfold equations. Repeated measures analysis of variance were performed to determine the systematic difference of whole-body and segmental body composition for different sex, lesion level and motor completeness subgroups.
Intraclass correlation coefficients (ICC) (two-way random, absolute agreement) were used to test the relative agreement in the body composition between DXA and other techniques. ICC values <0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.9 were considered indicative of poor, moderate, good, and excellent agreement, respectively [20]. To further characterize the absolute agreement between different techniques, Bland–Altman plots were created [21].
Prediction equations to estimate FFM and FM% in people with SCI were developed using multivariate regression analysis. FFM and FM% measured by DXA were used as dependent variables, impedance index (h2/Z) given by SF-BIA and MF-BIA, FM% and FFM (after calculated by total body mass) given by both skinfold equations, sex, lesion level, lesion completeness, and time since injury were used as independent variables. All independent variables were included separately at first and if they showed a p value below 0.1 the variables were added to the multivariate model. Backward elimination was used for the multivariate model until only significant determinants remained (p < 0.05). Bland–Altman plots were used to validate the new equations in the same group of participants as the equation developed by checking the systematic bias, mean difference and limits of agreement between the estimation of the new equations and the DXA outcomes (internal validation). All statistical analyses were performed using SPSS software (version 25, IBM, Somers, NY, USA). Bland–Altman plots were created using MedCalc (version 18.2.1, MedCalc Software Ltd, Ostend, Belgium). A p value < 0.05 was considered significant.
Results
Descriptives
Sixty-two percent of the participants were male, 65% had a paraplegia, and 52% had a motor complete injury. Due to the failed connection between the electrodes and the device and the input error of height, three participants were not able to complete the measurement with the SF-BIA. The calf circumference of one participant was not measured because the participant did not want to be measured at the lower limbs at that time. Table 1 shows descriptives of the total group and the subgroups.
SF-BIA and MF-BIA
Compared to DXA, both BIA methods had moderate to excellent relative agreement in estimating FFM, FM, and FM%. However, SF-BIA significantly underestimated while MF-BIA significantly overestimated FM% (Table 2). The mean difference of SF-BIA was −2.2% in estimating FM% with limits of agreement from −12.8 to 8.3%. These results are visualized in Bland–Altman plots with MF-BIA showing a proportional bias (p = 0.001) (Fig. 2).
Bland–Altman plots to show absolute agreement between percentage fat mass (FM%) measured by DXA and MF-BIA (a), SF-BIA (b), skinfold equations by Durnin–Womersley (c), and Goosey-Tolfrey et al. (d) in different sex, lesion level, and motor completeness subgroups. M male, F female, T tetraplegia, P paraplegia, C motor complete injury, and IC motor incomplete injury. DW skinfold equation by Durnin-Womersley, GT skinfold equation by Goosey-Tolfrey et al. The mean value of DXA and the other technique is on the x-axis, and the mean difference is on the y-axis. The solid line is the mean difference for each comparison. The dotted lines above and below the mean difference are the upper and lower limits of agreement. MF-BIA (p < 0.001, y = −9.1874 + 0.3978x, 95% CI of the slope, 0.2092–0.5864) and skinfold equation by Goosey-Tolfrey et al. (p = 0.005, y = 7.0788 − 0.3218x, 95% CI of the slope, −0.5410 to −0.1027) show a proportional bias with the 95% CI of the regression lines in the plots.
In the subgroups, compared to DXA, SF-BIA significantly underestimated, while MF-BIA significantly overestimated FM% in males, participants with paraplegia or with motor complete injury (Table 2). The relative agreement of BIA in estimating FM% in the subgroups is shown in Table 2.
Skinfold equations
Compared to DXA, the skinfold equations by Durnin–Womersley and Goosey-Tolfrey significantly underestimated FM%, which is also shown in the Bland–Altman plots, and showed a moderate relative agreement. The mean difference of Durnin–Womersley skinfold equation was −2.9% with limits of agreement from −12.6 to 6.7%. The skinfold equation by Goosey-Tolfrey showed a proportional bias (p = 0.005) (Fig. 2).
In the subgroups, compared to DXA, the Durnin–Womersley skinfold equation significantly underestimated FM% in males. In contrast, the Goosey-Tolfrey skinfold equation significantly underestimated FM% in females. Both skinfold equations significantly underestimated FM% in participants with paraplegia or with motor complete injury (Table 2). The relative agreement of skinfold equations in estimating FM% in the subgroups is shown in Table 2.
Segmental body composition
Compared to DXA, segmental MF-BIA significantly underestimated FFM in the arms and trunk and overestimated FFM in the legs.
In the subgroups, segmental MF-BIA significantly underestimated arms FFM in the males but not in females (males: p < 0.001; females: p = 0.08). It also showed a significant overestimation of legs FFM in males but not in females (males: p < 0.001; females: p = 0.996), a significant underestimation of arm FFM in both participants with tetraplegia and paraplegia (tetraplegia: p = 0.02; paraplegia: p < 0.001), a significant underestimation of trunk FFM in both participants with tetraplegia and paraplegia (tetraplegia: p < 0.001; paraplegia: p = 0.001), and a significant overestimation of leg FFM in participants with motor complete injury but not in participants with motor incomplete injury (motor complete: p < 0.001; motor incomplete: p = 0.67) (Table 3).
New equations for predicting FFM and FM%
Table 4 shows the included variables and the coefficient of determination (R2) of all the newly developed prediction equations for the general population of people with SCI and the internal validation results.
For predicting FFM, the new equation with the highest R2 included FFM calculated by Goosey-Tolfrey skinfold equation and total body mass and sex (R2 = 0.94), see Eq. 1 below.
The new equation for predicting FFM by SF-BIA included the impedance index (R2 = 0.83), see Eq. 2 below.
Since the impedance index given by BIA was not significant when predicting FM%, the new equations for predicting FM% were developed based on the two skinfold equations.
The new equation with the highest R2 included FM% estimated by Goosey-Tolfrey skinfold equation and sex (R2 = 0.66), see Eq. 3 below.
Discussion
According to our results, SF-BIA, MF-BIA, skinfold equations by Durnin–Womersley or by Goosey-Tolfrey et al. did not accurately estimate FM% in a general population of people with SCI. Among all the measurement techniques, SF-BIA showed the smallest mean difference in estimating FM% compared to DXA, but its large limits of agreement indicated the wide individual variation. Except for the estimation of skinfold equation by Goosey-Tolfrey et al. in males, the accuracy for estimation of FM% was better in females, participants with tetraplegia or with motor incomplete injury compared to their counterparts. New equations were developed for predicting FFM and FM% by SF-BIA, MF-BIA, and skinfold equations by Durnin–Womersley and Goosey-Tolfrey et al. showing good explained variances.
Based on impedance measures, BIA can estimate TBW from which FFM and FM are calculated [12]. Patel et al. reported that SF-BIA was a more accurate and less biased predictor of TBW than MF-BIA in critically ill participants [22]. This is in line with our results that SF-BIA showed better accuracy in estimating FM% than MF-BIA, which showed a proportional bias. An explanation for this result might be that impedance values do not appear to capture peripheral edema in a SCI group [23]. Although our study excluded patients with severe edema, MF-BIA results still showed that 74% (37 persons) of the participants were in an overhydrated state as they showed an ECW/TBW above 0.40 (ranged from 0.40 to 0.42) according to the commonly used cut-off value in different patient groups [24,25,26]. Since peripheral edema is a common clinical finding in people with SCI especially in the lower limbs due to the absence of the leg skeletal muscle pump [23, 27], it would be useful to measure the hydration status accurately in people with SCI and compare the accuracy of BIA in measuring body composition between people in an overhydrated state and people in a euhydration state. This could shed some light on whether the altered hydration in people with SCI might cause significant bias when measuring with BIA.
People with tetraplegia might have lower FFM and higher FM compared to able-bodied people and individuals with paraplegia because of the partial inability to use both arms independently for physical activity and food intake in daily life [13]. Therefore, a worse estimation by BIA in people with tetraplegia might be expected. However, we found that both BIA methods showed a better estimation in participants with tetraplegia than with paraplegia. This might be partly explained by the lesion characteristics of participants with tetraplegia. Among these participants (17 persons), five participants had a high lesion (between C3 and C5), but four of them were with a motor incomplete injury (AIS D). This finding should be confirmed in a larger sample of participants with tetraplegia and paraplegia, while the effect of hydration status in people with SCI should also be considered. Moreover, a motor complete injury might lead to a greater decline in FFM because of severe muscle atrophy compared to those with motor incomplete injury [13]. The muscle atrophy could change the body geometry such as the cross-sectional area of the limbs [3]. Since SF-BIA assumes that the body is a homogenous conductive cylinder of uniform length and cross-sectional area, the changes in body geometry may cause a significant measurement bias when using SF-BIA for people with motor complete injury. The body shape should be better represented by five interconnected cylinders (trunk, two legs, and two arms) as segmental MF-BIA supposes. The reason why segmental MF-BIA still showed a significant overestimation of FM% in motor completeness subgroups might also be related to the potential measurement bias due to the altered hydration status in people with SCI.
The segmental body composition outcomes estimated by segmental MF-BIA revealed similar trends as the whole-body composition outcomes, i.e., the estimation of segmental FFM tended to be better in females, participants with tetraplegia or with motor incomplete injury than in males, participants with paraplegia or with motor complete injury. This confirmed the previous findings that high relative errors for arms (13 to 17%) and legs (10 to 13%) FFM were found with segmental BIA measurements in able-bodied people [28, 29].
The equation by Goosey-Tolfrey included three more measurement sites together with calf circumference compared to the equation by Durnin–Womersley, taking more inter-individual variation in fat distribution into account, which might lead to a better estimation in males, participants with paraplegia or with motor incomplete injury [10]. Furthermore, unlike the Durnin–Womersley equation that was developed in able-bodied people, the Goosey-Tolfrey equation was developed in a group of wheelchair athletes that included 73% of people with SCI and the mean FFM of that group was similar to the mean FFM in our study. Those similarities between the two studies might explain the better estimation of the Goosey-Tolfrey skinfold equation. However, the Goosey-Tolfrey skinfold equation showed a proportional bias when estimating FM% compared to DXA, which indicated that it should be interpreted with caution when applying in the general SCI population. Moreover, our study found a larger bias, i.e., lower accuracy, in people with SCI when using the Durnin–Womersley skinfold equation in estimating FM% (mean difference: 2.9%; limits of agreement: −6.7 to 12.6%) compared to an able-bodied population (mean difference: 1%; limits of agreement: −7.1 to 8.5%) [30].
A possible explanation for why the Goosey-Tolfrey skinfold equation showed a better estimation in males than in females in our study might be that the equation was developed among male wheelchair athletes. Furthermore, as the Durnin–Womersley skinfold equation only included the measurement sites of upper extremities, the estimation might not entirely reflect the whole-body FM% in participants with paraplegia. The arms FFM in people with paraplegia could be even higher than able-bodied people while their legs FM% could be much lower [23]. Similarly, only including the measurement sites of upper extremities could lead to a significant underestimation of FM% in participants with motor complete injury due to the severe muscle atrophy in their lower extremities [3].
Our results are consistent with previous findings that BIA and skinfolds had large variability at the individual level when estimating FM% in wheelchair athletes [6, 10, 14]. Therefore, new equations to predict FFM and FM% in the general population with SCI were developed. Our new equations for predicting FFM and FM% by BIA and skinfold equations showed good explained variances with R2 above 0.6 and good internal validity with small mean differences and relatively narrow limits of agreement. However, as proportional bias was found in both MF-BIA and the Goosey-Tolfrey skinfold equation when estimating FM%, our new equations based on these techniques should be utilized with caution and further validated to investigate whether the new equations could be useful in clinical practice. When comparing the accuracy of similar equations derived by BIA in able-bodied people, we found similar R2 (able-bodied: 0.83–0.90; our new equations: 0.83–0.94), but the prediction error of able-bodied equations (2.9–3.9 kg) was smaller than our new equations (3.1–5.3 kg) [31]. It should be noted that our new equations should still be cross-validated in future studies, and the able-bodied equations were developed in a much larger sample with the age range from 12 to 94 years.
The strength of this study is that the comparisons between different measurement techniques were made in a more heterogeneous and representative SCI group, and can, therefore, be well-generalized to that population. A limitation is that our sample size did not allow us to cross-validate the new equations in a different group. Although our new equations showed good internal validity, the external validity of the new equations remains unclear. Furthermore, we did not measure the length of trunk and leg, disallowing the cross-validation for the prediction equation of TBW in people with paraplegia developed by Buchholz et al. which was reported to perform well in that group [12].
Since BIA and skinfold thickness measurements have shown high reliability in measuring able-bodied population [32, 33], they might still be useful tools in clinical practice if their reliability is high enough to monitor body composition changes over time in people with SCI. These techniques should be used and interpreted with caution regarding the individual variation resulted from personal and lesion characteristics. According to a previous study, it should also be noted that potential measurement errors might arise by DXA if the variation in soft tissue hydration was severe (perhaps in the range of 20–25% of total soft tissue mass), but this error is normally small under normal or even most clinical conditions and should not pose any substantial limitations to the accuracy of the DXA technique [34].
Future studies need to validate the new prediction equations from our study and investigate whether other techniques such as Bioimpedance spectroscopy may be more accurate in estimating whole-body and segmental body composition in people with chronic SCI. To our knowledge, the reliability of SF-BIA, segmental MF-BIA, and skinfold equations is population-specific and seems to be less reliable when estimating body composition in patients or elderly [32, 35]. Since no studies have tested the reliability of these techniques in people with SCI, studies with a large sample size are needed to investigate whether these techniques can be used to monitor longitudinal changes in body composition.
In conclusion, SF-BIA, segmental MF-BIA, skinfold equations by Durnin–Womersley or by Goosey-Tolfrey et al. did not accurately estimate FM% in a general SCI population. Although SF-BIA showed the smallest mean difference in estimating FM%, it should be used and interpreted with caution because of the wide individual variation. Except for the estimation of skinfold equation by Goosey-Tolfrey et al. in males, all the measurement techniques tended to show a better estimation of FM% in females, participants with tetraplegia or with motor incomplete injury than in males, participants with paraplegia or with motor complete injury. Future studies should investigate the reliability of BIA and skinfold equations and whether more accurate measurement techniques could be used when estimating whole-body or segmental body composition in the general SCI population. Besides, new prediction equations of FFM and FM% were developed with good explained variances. The new equations should be cross-validated in future studies.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile–Part I. J Spinal Cord Med. 2014;37:693–702.
Gater DR Jr. Obesity after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18:333–51.
Castro MJ, Apple DF Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80:373–8.
Silveira SL, Ledoux TA, Robinson-Whelen S, Stough R, Nosek MA. Methods for classifying obesity in spinal cord injury: a review. Spinal Cord. 2017;55:812–7.
Keil M, Totosy de Zepetnek JO, Brooke-Wavell K, Goosey-Tolfrey VL. Measurement precision of body composition variables in elite wheelchair athletes, using dual-energy X-ray absorptiometry. Eur J Sport Sci. 2016;16:65–71.
Willems A, Paulson TA, Keil M, Brooke-Wavell K, Goosey-Tolfrey VL. Dual-energy X-ray absorptiometry, skinfold thickness, and waist circumference for assessing body composition in ambulant and non-ambulant wheelchair games players. Front Physiol. 2015;6:356.
Eston RG, Rowlands AV, Charlesworth S, Davies A, Hoppitt T. Prediction of DXA-determined whole body fat from skinfolds: importance of including skinfolds from the thigh and calf in young, healthy men and women. Eur J Clin Nutr. 2005;59:695–702.
Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97.
Maggioni M, Bertoli S, Margonato V, Merati G, Veicsteinas A, Testolin G. Body composition assessment in spinal cord injury subjects. Acta Diabetol. 2003;40:S183–6.
Goosey-Tolfrey V, Keil M, Brooke-Wavell K, de Groot S. A comparison of methods for the estimation of body composition in highly trained wheelchair games players. Int J Sports Med. 2016;37:799–806.
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr. 2004;23:1226–43.
Buchholz AC, McGillivray CF, Pencharz PB. The use of bioelectric impedance analysis to measure fluid compartments in subjects with chronic paraplegia. Arch Phys Med Rehab. 2003;84:854–61.
Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN Jr, Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003;95:2398–407.
Mojtahedi MC, Valentine RJ, Evans EM. Body composition assessment in athletes with spinal cord injury: comparison of field methods with dual-energy X-ray absorptiometry. Spinal Cord. 2009;47:698–704.
Spungen AM, Bauman WA, Wang J, Pierson RN Jr. Measurement of body fat in individuals with tetraplegia: a comparison of eight clinical methods. Paraplegia. 1995;33:402–8.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34:535–46.
Kushner R, Schoeller DA, Fjeld CR, Danford L. Is the impedance index (ht2/R) significant in predicting total body water? Am J Clin Nutr. 1992;56:835–9.
Stewart A, Marfell-Jones M, Olds T, De, Ridder H. International society for the advancement of kinanthropometry: international standards for anthropometric assessment. Lower Hutt, New Zealand: International Society for the Advancement of Kinanthropometry; 2001.
Siri WE. The gross composition of the body. Adv Biol Med Phys. 1956;4:239–80.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63.
Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–10.
Patel RV, Peterson EL, Silverman N, Zarowitz BJ. Estimation of total body and extracellular water in post-coronary artery bypass graft surgical patients using single and multiple frequency bioimpedance. Crit Care Med. 1996;24:1824–8.
Cirnigliaro CM, La Fountaine MF, Emmons R, Kirshblum SC, Asselin P, Spungen AM, et al. Prediction of limb lean tissue mass from bioimpedance spectroscopy in persons with chronic spinal cord injury. J Spinal Cord Med. 2013;36:443–53.
Nishikawa H, Yoh K, Enomoto H, Ishii N, Iwata Y, Nakano C, et al. Extracellular water to total body water ratio in viral liver diseases: A study using bioimpedance analysis. Nutrients. 2018;10:1072.
Noda Y, Suzuki H, Kanai T, Samejima Y, Nasu S, Tanaka A, et al. The association between extracellular water-to-total body water ratio and therapeutic durability for advanced lung cancer. Anticancer Res. 2020;40:3931–7.
Sukackiene D, Laucyte-Cibulskiene A, Vickiene A, Rimsevicius L, Miglinas M. Risk stratification for patients awaiting kidney transplantation: role of bioimpedance derived edema index and nutrition status. Clin Nutr. 2020;39:2759–63.
Seem E, Stranden E, Stiris MG. Computed tomography in deep venous thrombosis with limb oedema. Acta Radio Diagn. 1985;26:727–30.
Tagliabue A, Andreoli A, Comelli M, Bertoli S, Testolin G, Oriani G, et al. Prediction of lean body mass from multifrequency segmental impedance: influence of adiposity. Acta Diabetol. 2001;38:93–7.
Bracco D, Thiebaud D, Chiolero RL, Landry M, Burckhardt P, Schutz Y. Segmental body composition assessed by bioelectrical impedance analysis and DEXA in humans. J Appl Physiol. 1996;81:2580–7.
Leahy S, O’Neill C, Sohun R, Toomey C, Jakeman P. Generalised equations for the prediction of percentage body fat by anthropometry in adult men and women aged 18-81 years. Br J Nutr. 2013;109:678–85.
Sun SS, Chumlea WC, Heymsfield SB, Lukaski HC, Schoeller D, Friedl K, et al. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. 2003;77:331–40.
Aandstad A, Holtberget K, Hageberg R, Holme I, Anderssen SA. Validity and reliability of bioelectrical impedance analysis and skinfold thickness in predicting body fat in military personnel. Mil Med. 2014;179:208–17.
Buckinx F, Reginster J-Y, Dardenne N, Croisiser J-L, Kaux J-F, Beaudart C, et al. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: a cross-sectional study. BMC Musculoskelet Disord. 2015;16:1–7.
Pietrobelli A, Wang Z, Formica C, Heymsfield SB. Dual-energy X-ray absorptiometry: fat estimation errors due to variation in soft tissue hydration. Am J Physiol-Endocrinol Metab. 1998;274:E808–16.
Sergi G, Lupoli L, Enzi G, Volpato S, Perissinotto E, Bertani R, et al. Reliability of bioelectrical impedance methods in detecting body fluids in elderly patients with congestive heart failure. Scand J Clin Lab Investig. 2006;66:19–30.
Acknowledgements
We thank Amsterdam University of Applied Sciences (Dr. Meurerhuis) and the rehabilitation center Reade in Amsterdam which have contributed to the organization of measurements. We appreciate the cooperation of all the participants, the master students who helped with the measurements and Drs. Robert G. Memelink for providing DXA training and guidance.
Author information
Authors and Affiliations
Contributions
SDG conceived the study. WA and JA helped with the recruitment of participants. YM collected and analysed the data. YM, SDG, PW and TJ interpreted the data. YM drafted the paper. YM, SDG, PW and TJ revised the paper and all authors approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Medical Ethical Committee of Slotervaart hospital and Reade (NL64704.048.18).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, Y., de Groot, S., Weijs, P.J.M. et al. Accuracy of bioelectrical impedance analysis and skinfold thickness in the assessment of body composition in people with chronic spinal cord injury. Spinal Cord 60, 228–236 (2022). https://doi.org/10.1038/s41393-021-00682-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00682-w
This article is cited by
-
The effect of breathing exercises and mindset with or without cold exposure on mental and physical health in persons with a spinal cord injury—a protocol for a three-arm randomised-controlled trial
Spinal Cord (2024)
-
Predicting resting energy expenditure in people with chronic spinal cord injury
Spinal Cord (2022)