Abstract
One of the many challenges with necrotizing enterocolitis (NEC) remains our inability to make an accurate diagnosis of NEC. The lack of a unifying cause and multiple variations in presentations lead to great uncertainty with NEC. Separating out the needs of the researcher wanting to define NEC from the clinician and patient family’s perspectives who want an accurate diagnosis for NEC is important. The need to augment and/or replace the outdated modified Bell staging criteria is crucial to improving NEC management. Emerging literature suggests that genetic susceptibility and stool microbiota signatures may help identify preterm infants at increased risk of the disease. Ongoing studies using single or multi-omic approaches may help to characterize biomarkers that will aid in the prediction or early diagnosis of NEC, as well as differentiate other causes of severe bowel injury. Bowel ultrasound shows promise in improving our diagnostic accuracy for NEC but has been slow in adoption. Patient family perspectives are key in accelerating our efforts to integrate newer diagnostic methods into practice.
Similar content being viewed by others
Introduction
To the clinician, the classic diagnosis of necrotizing enterocolitis (NEC) has been based on the observation of nonspecific signs of systemic inflammation and local abdominal signs along with radiographs to support the presence of gastrointestinal inflammation. When NEC is suspected, the staging of NEC has benefited from the modified Bell staging criteria to help support clinical management. However, there are numerous shortcomings in the current use of Bell’s criteria.1,2 Importantly, it was not developed as an early preclinical diagnostic tool but useful when NEC has already occurred. The features of Bell’s criteria represent clinical, laboratory, and radiographic signs most of which are non-specific and sometimes insensitive. For example, systemic signs can include apnea, respiratory failure, lethargy, poor feeding, or temperature instability, but each of these may be part of other clinical entities where well-being is compromised. Even more severe markers of hemodynamic compromise such as shock are also not specific to NEC. The classic radiographic sign of intestinal pneumatosis on serial abdominal radiographs are frequently overread from patterns of gas in stool. Making a diagnosis of NEC is complex due to the absence of a single pathognomonic sign or test. The most definitive way to make the diagnosis of NEC is histologically either through surgery or postmortem. About a third of infants require surgery, so for most infants the other signs of NEC need to be weighed in. A majority of clinicians continue to base their diagnosis using Bell’s criteria even though they recognize its pitfalls. This is largely due to the lack of any reasonable substitute of these outdated diagnostic criteria that has readily supplanted it in the clinical arena.
Defining versus diagnosing NEC
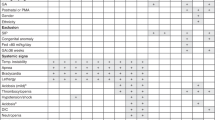
Some of the uncertainty in the diagnosis of NEC may arise from the different perspectives between researchers who want to define NEC and clinicians who need to diagnose NEC (see Table 1). Researchers want a better understanding of NEC through data collection around a specific case definition while also gathering data regarding risk factors and pathogenesis of NEC. The clinician’s aim is to be able to diagnose NEC but they also need to take care of cases of bowel injury that are not NEC. Modi et al. have evaluated a broader gestational age-specific case definition to identity risk stratification.3 The NEC researcher is looking for predictive factors and mechanisms for possible therapies for NEC and leans toward a consistent and tight definition so that comparisons across centers can be done readily. Gordon et al. describes that we have been able to effectively separate out spontaneous intestinal perforation (SIP) from NEC but that there are many other subgroups within what we call NEC that may have differing mechanisms.4 These newer definitions do not readily lend themselves to pragmatic clinical care algorithms and may be reasons why the modified Bell’s criteria has remained dominant for so long. The clinician and patient families also want a clear and consistent way to diagnose NEC but that includes being taken care of even if the patient does not conveniently fit into a working definition. For example, recently there has been another effort to define NEC with a gestational age and postnatal time limited definition.5 In this model, infants diagnosed with NEC prior to 10 days of life would not be defined as classic NEC. This tighter definition may improve the consistency of the recruited population for research purposes to identify more homogeneous cases, but for the patient who does not meet this definition of NEC, this could leave their family feeling abandoned. The clinician and patient families desire a clear way to diagnose NEC for their patients so that not only management but also prognosis can be determined. Also, collecting additional data around a case to support building a better understanding of the pathophysiology of NEC may not immediately help the individual patient, their family, or neonatal care team. It is imperative then that the goals and objectives of the clinician and NEC researchers are shared with each other and with patient families who may be unaware of these differing perspectives in our understanding of this complex condition.
Why is it so challenging to make a diagnosis of NEC?
The primary reason it is so challenging is that we remain without a good model of NEC pathogenesis that can separate out the various phenotypic presentations on preterm gut injury that is commonly grouped as NEC (Fig. 1). In 20–30% of infants who develop NEC, positive blood cultures can be identified.6 There are several key elements that are defined risk factors for the development of NEC. The first is the predominance of NEC in preterm infants. The understanding that preterm infants have immature gastrointestinal tract supporting the largest immune and nervous system of the body makes it a likely determinant of the resulting bowel injury and inflammation in NEC. Further, the presence of feeding in almost all cases of preterm NEC suggests that enteral substrate is required and that the strain of feeding placed on the gastrointestinal tract are important to the development of NEC. A study by Berseth et al. suggested that an initial period of trophic feeding compared to an immediate feed advancement could lower the risk of NEC in preterm infants.7 The overwhelming evidence that mothers’ milk feeding leads to a dose-dependent lowering of risk for NEC suggests that feeding alone is not completely the dependent factor.8 Rather, recently recognized identification of altered gut microbiota in preterm infants and a distinct difference in those infants who ultimately develop NEC suggests strongly that dysbiosis is another requirement for NEC.9
Diverse intestinal injury phenotypes in the preterm gut can be classified NEC. Granularity of current diagnostic criteria for NEC does not allow distinguishing various forms of intestinal injury. Phenotypes are clustered based on pathogenesis, with proximity and remoteness implying similar or dissimilar mechanisms. Darker shadowed boxes represents more severe phenotypes. TANEC transfusion-associated NEC, GIT gastrointestinal tract, SIP spontaneous ileal perforation, NIRS near-infrared spectroscopy, Hgb hemoglobin, SMA superior mesenteric artery.
Timing of NEC
Clues may be discovered from the timing of NEC across gestational age. The most immature infants develop NEC at a later postnatal age than more mature preterm infants.10,11 Term NEC infants also present much earlier in comparison.12,13 This suggests several possibilities as to cause. The first is that the very immature infants advance in feeding slower than moderate preterm or term infants. This is consistent with clinical experience in that many cases of NEC develop when higher feeding volumes have been attained. These volumes may then provide more substrate for microbes to thrive as well as put more stress on the gut to propel luminal content, digest and absorb nutrients, and control the fermentation of microbes. The second and not exclusive possibility is that the timing of NEC occurs at a critical developmental window of susceptibility coinciding with dysregulated intestinal or systemic immune responses skewed toward exaggerated inflammatory responses to bacteria or pathogens. Infants generally develop NEC closer to 30–32 weeks of corrected gestational age and this period may reflect greater sensitivity to aberrant inflammation.
Developing a new diagnostic model for NEC
In developing a model for diagnosing NEC, we need to recognize that the final pathway of clinical signs (abdominal and systemic signs) and hallmark findings of gut injury and bacterial invasion (pneumatosis intestinalis) can be arrived from different pathways (Fig. 1). Other pathways may lead to similar intestinal injury found in classical NEC and may have different pathophysiologic mechanisms. These may arise from identified infections, including viral causes, ischemia–reperfusion-related injury, congenital anomalies such as gastroschisis or intestinal atresia, cow’s milk allergies, or relate to hyperosmolar feeds that cause mucosal injury. Developing tests to screen for these separate conditions may differ from that of classical NEC. Together, these results would better inform how to manage individual patients with suspected NEC and has implications for long-term prognosis.
As an example of separation, we now recognize that SIP is a distinct condition that does not appear to revolve around feeding and dysbiosis but rather extreme prematurity and the loss of structural integrity of the bowel wall resulting in the dilatation and perforation of vulnerable segments that may be more likely to be compromised in blood supply.14,15 SIP can be present even in the absence of inflammation, which is an important clue. In addition, SIP occurs in the absence of radiographic findings of pneumatosis intestinalis, transient thickening of the intestinal wall, and fixed dilated small bowel loops. In the past, overreading of abdominal radiographs with possible pneumatosis intestinalis or detection of some histologic inflammation often after a prolonged period of perforation may have classified cases of SIP as NEC. Our ability to refine further the markers of disease in all spheres, clinical, laboratory, and radiographic, is important in improving our understanding of neonatal bowel injury as it relates to providing more tailored management.
The rapidity of onset of NEC is a barrier
The onset of NEC can be likened to early-onset sepsis as the development of sepsis with severe consequences can be rapid and lethal. Markers of early sepsis have been developing with high-resolution monitoring of vital signs that can be analyzed to identify warning signs hours before more clinical signs appear.16,17 With NEC, no early biomarkers have reached the point that can assist the clinician yet, particularly in light of the rapidity of onset in the disease. The difference between NEC and sepsis is that with sepsis a defined microbe can be identified as the causative agent of illness, whereas with NEC there is no such singular cause. The majority of preterm infants who develop NEC are healthy, feeding well, and growing.18 The rapidity of onset and degree of severity of NEC makes it particularly challenging to find an early diagnostic marker for NEC. Active research continues to define new biomarkers that have the positive and negative predictive values to alter clinical care with NEC.19
There are a number of studied biomarkers that may assist in early prediction of NEC, diagnosing NEC, and/or determining the severity of NEC, but none have become part of clinical practice or a standard definition.20,21,22 One of the biggest challenges is that many promising biomarkers target factors that are elevated during inflammation that are also present in the face of sepsis and ileus without NEC and may not provide good specificity.21
Harm of overdiagnosis
With nonspecific clinical signs and a suggestive radiograph with possible pneumatosis, clinicians may be inclined to treat for the suspicion of NEC. Due to the combination of the imprecision of our diagnostic skills and tools and the fear of missing the rapid development of NEC, it is highly likely that we are overtreating many of our infants, particularly in those who are born premature. This is not a benign overreach in practice as the consequences of treating for NEC involves blood work, coverage with broad spectrum antibiotics, and arrest of any enteral feeds. Many infants then may be subject to corresponding blood loss setting them up for greater risk of anemia, further derangement of their gut microbiome, and loss of growth potential. The irony of these consequences is that anemia, dysbiosis, and lower doses of mothers’ milk intake are all known to increase the chances of NEC development. It is crucial then to develop better strategies to recognize what is not NEC to avoid overtreatment.
Genetics of NEC
The premise for NEC having a genetic predisposition is founded by twin studies suggesting increased concordance in identical twins, increased incidence in African American infants, and the observation that the presence of clinical risk factors or gut dysbiosis patterns does not predict NEC development robustly.11,23,24 Unlike single-gene disorders, NEC is a complex disease, where interactions between genetic factors, gut microbes, and intestinal injury likely programs susceptibility and severity. With NEC, the repertoire of responses to gut microbes, injury, and/or their ability to generate an inflammatory response is likely to be highly variable between individuals and thereby determine variability in disease susceptibility or severity. Several investigators have attempted to identify possible genes, mutations/variants in which program increased or decreased host susceptibility to NEC.25 Most of these studies have been underpowered, have not probed functionality of causal variants, and remain to be replicated.25 However, consistent with mechanistic animal studies and descriptive studies using human pathological samples, there is an emerging signature for defects in host innate immune response genes as potential NEC susceptibility loci.25,26 Large studies or sequencing-based pilot studies with functional analysis have identified NOD2 (nucleotide-binding oligomerization domain containing 2) and SIGIRR (single immunoglobulin interleukin-1 related receptor), genes that regulate intestinal innate immune signaling, as potential loci for NEC.25,27,28 Interestingly, a genetic variant in ATG16L1, implicated in adult inflammatory bowel disease was associated with less NEC in a study enrolling >1000 infants.25 Considering the genetic heterogeneity likely to underpin NEC susceptibility and role of rare genetic variants in complex disease susceptibility, targeting candidate pathways or unbiased “genome-wide sequencing approaches will be required to uncover novel candidate genes and pathways for NEC susceptibility.” “Emphasis on precise phenotype definition, adequate validation cohorts, and functional analysis” will improve our precision in identifying robust genetic risk factors for NEC. Unlike other biomarkers, which may vary with gestational age or maturity, genetic risk factors remain static and can be identified within days of birth allowing for prevention-based approaches.25 In addition to future potential use for screening babies, genetic loci can potentially reveal novel insights to disease pathogenesis informing therapeutic approaches for prevention.25,26,27,28 Finally, “combining genetic approaches with functional genomics and gut microbiome analysis is likely needed to comprehensively decipher NEC susceptibility and potentially define the molecular basis of the NEC, as it is a complex phenotype with gene–environment–microbial interactions”.25
Role of imaging in the diagnosis of NEC
One of the key shortcomings with clinical diagnosis of NEC has been the weakness of plain abdominal radiographs (AR) to be sensitive and specific for identifying different stages of NEC. AR can show key aspects of bowel injury that are generally specific when found. However, there are other findings that are not specific or sensitive enough to diagnose NEC. Frank perforation particularly in very small preterm infants can be readily missed on radiographs that do not show free air or pneumoperitoneum. This insensitivity is a challenge to surgeons who need to make the call to operate.
One of the most promising aspects of refining the diagnosis of NEC lies in the use of abdominal ultrasound to image bowel dynamically. Bowel ultrasonography (BUS) has been shown to be potentially useful in the diagnosis of NEC.25,29 A number of benefits have been shown by these studies so far (listed in Table 2), including identifying unique features of bowel wall architecture (thickness and echogenicity, pneumatosis intestinalis) and function (motility and blood perfusion) (Table 3).
The first descriptions of potential value of BUS in NEC showed in the literature almost 15 years ago, but there has been very little uptake in these techniques for neonatal care.30 This lag has been at both the level of the radiology and neonatal communities. The radiology community has not added these technical skills of neonatal bowel assessment and/or trained their technicians. There still needs to be clear protocols established before these practices can disseminate broadly. Furthermore, the neonatal community has been also slow in integrating point-of-care ultrasound into provider-based practice compared to other specialties, such as emergency medicine and pediatric critical care. Even those neonatal providers who do practice point-of-care ultrasound have not learned imaging techniques for bowel.
Further clinical evidence to define specific BUS benefits may support improved adoption. These include studies to help to determine optimal surgical management (timeliness, avoidance, etc.), value in reducing excessive treatment for suspected NEC, guiding recommencement of feeding after suspected or confirmed NEC, and measuring combined benefits with plain radiographs. It may be possible to modify or create an image-based diagnostic criteria in conjunction with newer biomarkers to further clarify disease presence.
Patient family perspective
Incorporating the parent’s perspectives in disease diagnosis may be an important driver for progress. Parents lament when NEC occurs to their child without being adequately informed about their child’s risk of the disease or the associated protective and risk factors. Parents often learn about NEC and the severity of the condition when their child is actually being diagnosed. This lack of information and the uncertainty around diagnosis are sources of profound stress to patient families. Knowing who is at highest risk and stratifying our management based on risk would help target infants developing NEC. Patient families and clinicians experience feelings of intense helplessness, discontent, and frustration by the inability to improve outcomes once NEC occurs. Accordingly, the primary effort remains focused on disease prevention. Parents need to be informed about the lack of reliable early biomarkers for NEC and the urgent need for precise tests to identify the earliest signs of NEC so that rapid intervention can occur before disease progression. There is an opportunity for patient families to engage in research and advocacy efforts that can help to advance the development, adoption, and integration into clinical practice of improved diagnostic approaches to NEC, such as BUS.
Conclusion
There is an urgent need to support the neonatal clinician in making a timely and accurate diagnosis of NEC. Bell’s criteria is outdated but suitable alternative definitions have not been integrated clinically. Separating out SIP and conditions mimicking classic NEC can help differentiate our clinical strategies. Identification of robust genetic biomarkers will represent a significant advance as this will not only allow screening-based identification of high-risk infants but potentially provide a tool for classifying NEC or defining it. When combined with measures of gut dysbiosis and clinical risk factors, it could lead to the development of precision approaches for risk stratification, targeted intervention, and precision therapy. Future integration of more visually accurate modes of imaging such as BUS may also increase our ability to recognize those infants at highest risk of NEC.
References
Neu, J. Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr. Clin. North Am. 43, 409–432 (1996).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
Battersby, C., Longford, N., Costeloe, K., Modi, N. & Group UKNCNES. Development of a gestational age-specific case definition for neonatal necrotizing enterocolitis. JAMA Pediatr. 171, 256–263 (2017).
Gordon, P. V., Swanson, J. R., MacQueen, B. C. & Christensen, R. D. A critical question for NEC researchers: can we create a consensus definition of NEC that facilitates research progress? Semin. Perinatol. 41, 7–14 (2017).
Caplan, M. S. et al. Necrotizing enterocolitis: using regulatory science and drug development to improve outcomes. J. Pediatr. 212, 208.e1–215.e1 (2019).
Kliegman, R. M. & Fanaroff, A. A. Necrotizing enterocolitis. N. Engl. J. Med. 310, 1093–1103 (1984).
Berseth, C. L., Bisquera, J. A. & Paje, V. U. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 111, 529–534 (2003).
Meinzen-Derr, J. et al. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J. Perinatol. 29, 57–62 (2009).
Neu, J. & Pammi, M. Necrotizing enterocolitis: the intestinal microbiome, metabolome and inflammatory mediators. Semin. Fetal Neonatal Med. 23, 400–405 (2018).
Yee, W. H. et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 129, e298–e304 (2012).
Uauy, R. D. et al. Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. National Institute of Child Health and Human Development Neonatal Research Network. J. Pediatr. 119, 630–638 (1991).
Maayan-Metzger, A., Itzchak, A., Mazkereth, R. & Kuint, J. Necrotizing enterocolitis in full-term infants: case-control study and review of the literature. J. Perinatol. 24, 494–499 (2004).
Li, Q. Y. et al. Differences in the clinical characteristics of early- and late-onset necrotizing enterocolitis in full-term infants: a retrospective case-control study. Sci. Rep. 7, 43042 (2017).
Vongbhavit, K. & Underwood, M. A. Intestinal perforation in the premature infant. J. Neonatal Perinat. Med. 10, 281–289 (2017).
Gordon, P. V. & Attridge, J. T. Understanding clinical literature relevant to spontaneous intestinal perforations. Am. J. Perinatol. 26, 309–316 (2009).
Moorman, J. R. et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J. Pediatr. 159, 900–906 e901 (2011).
Fairchild, K. D. et al. Vital signs and their cross-correlation in sepsis and NEC: a study of 1,065 very-low-birth-weight infants in two NICUs. Pediatr. Res. 81, 315–321 (2017).
Kliegman, R. M., Walker, W. A. & Yolken, R. H. Necrotizing enterocolitis: research agenda for a disease of unknown etiology and pathogenesis. Pediatr. Res. 34, 701–708 (1993).
Wang, K., Tao, G., Sun, Z. & Sylvester, K. G. Recent potential noninvasive biomarkers in necrotizing enterocolitis. Gastroenterol. Res. Pract. 2019, 8413698 (2019).
Garg, B. D., Sharma, D. & Bansal, A. Biomarkers of necrotizing enterocolitis: a review of literature. J. Matern. Fetal Neonatal Med. 31, 3051–3064 (2018).
Gephart, S. M. et al. Changing the paradigm of defining, detecting, and diagnosing NEC: perspectives on Bell’s stages and biomarkers for NEC. Semin. Pediatr. Surg. 27, 3–10 (2018).
Goldstein, G. P. & Sylvester, K. G. Biomarker discovery and utility in necrotizing enterocolitis. Clin. Perinatol. 46, 1–17 (2019).
Zani, A. & Pierro, A. Necrotizing enterocolitis: controversies and challenges. F1000Res 4, 1373 (2015).
Bhandari, V. et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics 117, 1901–1906 (2006).
Cuna, A., George, L. & Sampath, V. Genetic predisposition to necrotizing enterocolitis in premature infants: current knowledge, challenges, and future directions. Semin. Fetal Neonatal Med. 23, 387–393 (2018).
Nino, D. F., Sodhi, C. P. & Hackam, D. J. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 13, 590–600 (2016).
Sampath, V. et al. SIGIRR genetic variants in premature infants with necrotizing enterocolitis. Pediatrics 135, e1530–e1534 (2015).
Hartel, C. et al. NOD2 loss-of-function mutations and risks of necrotizing enterocolitis or focal intestinal perforation in very low-birth-weight infants. Inflamm. Bowel Dis. 22, 249–256 (2016).
Kim, J. H. Role of abdominal US in diagnosis of NEC. Clin. Perinatol. 46, 119–127 (2019).
Faingold, R. et al. Necrotizing enterocolitis: assessment of bowel viability with color doppler US. Radiology 235, 587–594 (2005).
Acknowledgements
V.S. is supported by 1R01DK117296-A1 from the National Institutes of Health and a Katharine Berry Richardson Award. Publication of this article was sponsored by the Necrotizing Enterocolitis (NEC) Society, Patient-Centered Outcomes Research Institute, and National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to conception of the article, drafting the article, critical revisions, and approval of the final version to be published.
Corresponding author
Ethics declarations
Competing interests
J.H.K. received grant support from Mallinckrodt and Fresenius-Kabi; received consultant or advisor fees from Evolve Bioscience, Medela, Alcresta, and Ferring; received lecture fees from Mead Johnson Nutrition and Abbott Nutrition; owns shares in Nicolette and Astarte Medical; served as expert witness; and holds a patent for a newborn heart rate device. None of these entities or funding bodies had any role in this manuscript. J.C. is the founder and Director of The NEC Society, sponsor of the supplement.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, J.H., Sampath, V. & Canvasser, J. Challenges in diagnosing necrotizing enterocolitis. Pediatr Res 88 (Suppl 1), 16–20 (2020). https://doi.org/10.1038/s41390-020-1090-4
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-1090-4
This article is cited by
-
Comparison of preoperative and intraoperative surgeon diagnosis and pathologic findings in spontaneous intestinal perforation vs necrotizing enterocolitis
Journal of Perinatology (2024)
-
Novel pathogenic GATA6 variant associated with congenital heart disease, diabetes mellitus and necrotizing enterocolitis
Pediatric Research (2024)
-
Artificial intelligence to classify acquired intestinal injury in preterm neonates—a new perspective
Pediatric Research (2024)
-
Point-of-care abdominal ultrasound in pediatric and neonatal intensive care units
European Journal of Pediatrics (2024)
-
Reduction in regulatory T cells in preterm newborns is associated with necrotizing enterocolitis
Pediatric Research (2023)