Abstract
Cardiovascular health of premature infants reaching early adulthood is an area of ongoing research. There is emerging evidence delineating the challenges faced by those individuals. Young adults born premature demonstrate a unique cardiac phenotype characterized by reduced biventricular volume, relatively lower systolic and diastolic function, and a disproportionate increase in muscle mass. This may clinically manifest by an increased risk of cardiovascular incidents, hypertension, and reduced exercise tolerance. Those consequences appear to result from early postnatal cardiac remodeling due to premature birth and associated co-morbidities. Recent evidence suggests that early exposure to breast milk slows down or even arrests those pathophysiological changes, thereby mitigating the long-term adverse effects of premature birth on cardiovascular health. In this review article, we discuss the role of breast milk in preventing early adulthood cardiovascular disease in infants born premature. We explore the emerging evidence and examine the possible mechanistic pathways mediating this phenomenon. Furthermore, we aim to demonstrate the vital role of early breast milk exposure in preventing cardiovascular disease in preterm infants.
Similar content being viewed by others
Introduction
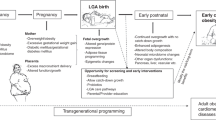
Improved survival of extremely premature infants has led to increased recognition of the importance of deciphering cardiovascular health beyond the neonatal period. Young adults born premature demonstrate a unique cardiac phenotype characterized by reduced biventricular volume, relatively lower systolic and diastolic function, and a disproportionate increase in muscle mass.1,2 Early exposure to breast milk may have protective effects by arresting those pathophysiological changes, potentially dampening the development of cardiovascular disease risk in adulthood.3 This growing knowledge of human milk composition has fostered an enhanced appreciation of its potential role in short- and long-term cardiovascular health and may provide insight into the mechanism by which human milk acts a protective cardiovascular agent. In this review, we discuss the components of breast milk that may play a role in preventing early adulthood cardiovascular disease in individuals born premature; we explore the current state of the science and discuss the gaps in knowledge; we examine possible mechanistic pathways mediating this phenomenon; and offer recommendations for research opportunities (Fig. 1).
Key components of breast milk
Human milk is a dynamic, multidimensional liquid that delivers the standard source of nutrition to infants.4 Sustained mothers’ own milk feedings decrease the incidence and severity of several common neonatal complications of prematurity including necrotizing enterocolitis, sepsis, retinopathy of prematurity, bronchopulmonary dysplasia (BPD), feeding intolerance, and neurodevelopmental impairment.5,6,7,8 Similarly, children and young adults born preterm who received human milk during the neonatal period are often less susceptible to infection and metabolic disorders (e.g., obesity and/or diabetes) and may have improved cardiovascular outcomes in later life.3,9,10,11,–12 Breast milk contains a high bioactivity and bioavailability of growth factors, enzymes, antibodies, and stem cells that may directly contribute to improved cardiovascular development during the neonatal and infant stages of maturation, which are not found in infant formulas. Comprehension of the major determinants of human milk composition and identification of confounders that may contribute to the differences is a prerequisite to exploring the mechanistic link between early-life exposure and the long-term cardiovascular benefits.
Colostrum is the human milk that is produced in the immediate postnatal period. It contains immune-protective factors (e.g., secretory immunoglobulin (IgA), lactoferrin, leukocytes, epidermal growth factor, etc.), which are more highly concentrated in the colostrum of mothers who deliver preterm infants.3,11,12,13,–14 Early provisions of colostrum lead to sustained administration of mothers’ own milk in preterm infants through hospital discharge.15 In contrast to mature breast milk, colostrum contains relatively low concentrations of lactose, suggesting its primary function to be immunologic and trophic rather than nutritional.16 The changeover to transitional milk may be delayed in preterm infants beyond 72 h of age. Although the transitional milk shares some of the characteristics of colostrum, it reflects a period of increased milk production over the first 2 weeks of age to support the nutritional and developmental needs of the infant. By 1 month of age, human milk is considered fully mature and is rich in macronutrients and micronutrients and remains relatively similar in composition.17 Mature milk is less concentrated than transitional milk, and its lower nutrient density is maintained throughout the first post-partum year.17
The mean macronutrient composition of mature, term milk is significantly different than preterm milk and donor milk based on its protein, fat, and lactose concentrations. The protein content of milk obtained from mothers who deliver preterm is significantly higher than that of mothers who deliver at term. Protein levels decrease in human milk over the first 4–6 weeks of age, regardless of timing of delivery. Preterm infants need protein supplementation as the declining levels are limiting in this population. Human milk fat is characterized by high contents of palmitic and oleic acids, with fat as the most highly variable macronutrient of milk.18 Hindmilk, defined as the last milk of a feed, may contain 2–3 times the concentration of milk fat found in foremilk and is more energy dense because of its higher lipid. Interestingly, approximately 25% of the variation in lipid concentration between mothers’ milk may be explained by maternal protein intake.17 The principal carbohydrate of human milk is lactose. The concentration of lactose in human milk is the least variable of the macronutrients, but this disaccharide is relatively low in colostrum and increases over time with more mature milk. Complex oligosaccharides, or human milk oligosaccharides (HMOs), are the second most abundant carbohydrate in human milk but highly variable with differences between populations and over time.19 HMO function as prebiotic agents, act as a decoy, and provide a source of fructose and sialic acid, which are important in host defense and neurodevelopment.20 Premature milk has higher concentration of HMO than term milk.21 In addition, it is also richer in glycosaminoglycans that also act as a decoy by providing binding sites for pathogenic bacteria to prevent adherence to the enterocyte.22
Micronutrients will differ in human milk contingent on maternal diet and body stores and include, but are not limited to, vitamins A, B, D, E, and K.23 Human milk also contains a variety of bioactive factors that come from a multitude of sources and also help to explain the variability in milk concentrations. In addition to the macronutrients and micronutrients, the bioactive factors with protective properties (e.g., immunological, anti-inflammatory, antioxidant, intestinal growth-enhancing, hormones, and epigenetic modulators) have wide-ranging effects on the intestinal tract, vasculature, nervous system, and endocrine system that are important components of the innate immune system.17 Specific bioactive molecules which are expanded on below that have been implicated in long-term cardiovascular outcomes include insulin-like growth factors, vascular endothelial growth factors, and microRNAs.24,25 While differences in cytokines, growth factors, and lactoferrin between preterm and term milk are most dramatic in colostrum and early milk, they mostly resolve by 1 month of age.
Association of breast milk exposure with long-term cardiovascular health of premature neonates
It is now well-established that, in comparison to age-matched term-born counterparts, children and adults who were born prematurely are at higher risk of long-term health complications affecting various organ systems.26 From a cardiovascular health perspective, large population-based studies have demonstrated higher incidence of ischemic heart disease in adults who were born premature,27 while a number of individual cohort studies and systematic reviews found an increased prevalence of cardio-metabolic risk factors in children and young adults who were born premature.26 These include higher systolic and diastolic blood pressures, increase in the incidence of hypertension, higher insulin resistance and lower glucose tolerance, possibly abnormal lipid profile, and structural changes in the cardiovascular system, such as lower arterial distensibility, increased arterial wall thickness, and changes in size and shape of cardiac chambers. Among these, the modest but significant increase in blood pressure in premature-born children and adults was the most consistent findings between various cohorts.12
An emerging body of literature exists regarding long-term cardiovascular health of ex-premature infants, but there is lack of robust data regarding its relationship with the quality and quantity of nutritional exposure during initial hospital stay. The publications regarding this association are primarily provided by the same group of investigators through sequential follow-up studies of a unique cohort of premature infants.3,28,29,30,31,–32 The original cohort consisted of 926 preterm neonates, born with birth weight <1850 g, who participated in 2 parallel trials of nutritional interventions at 5 tertiary neonatal centers in the UK between 1982 and 1985. One trial compared the use of banked pasteurized donor breast milk (DBM) vs. nutrition-enriched preterm formula, while the other tested standard term formula vs. the same preterm formula.33 The infants were randomized to their assigned group within 48 h of birth and continued to receive the intervention until they were 2000 g in weight or discharged. In each of these trials, depending on the mother’s wish to provide expressed breast milk (EBM), infants were randomized to receive the assigned diet exclusively or as a supplement to EBM. Overall, this methodology resulted in creation of the following unique groups of patients based on nutritional exposure: DBM+/−EBM (exclusive human milk diet), standard or preterm formula (exclusive formula diet), and those who received a combination of formula and human milk in various proportions. These cohorts were longitudinally followed up at various time points and assessed for cardiovascular health using different surrogate markers, which provided interesting and conflicting results. Three hundred and forty seven of these infants had their blood pressure measured using a conventional sphygmomanometer at 18 months’ corrected age.29 The investigators found no difference between infants who received DBM or standard formula, as well as between groups who received a high sodium diet (preterm formula) vs. low sodium diet (DBM or standard formula), either with or without mother’s EBM. Subsequently, 758 children from the original cohorts (99% of survivors) had their blood pressure measured again at 7.5–8 years of age. On this occasion, measurements were done using an automated device in 72% of subjects, with the conventional method used in the rest.28 Small differences in values obtained were accounted for in the analyses. Despite significant inter-group differences in the intake of macronutrients and major variations in postnatal growth during hospital stay, no difference was found in systolic or diastolic blood pressures between children who received exclusive DBM vs. preterm formula (95% confidence interval (CI) for mean difference in mm Hg: −3.0 to 3.2 and −3.2 to 2.0 for systolic and diastolic measurements, respectively), DBM+EBM vs. preterm formula+EBM (95% CI for mean difference in mm Hg: −2.2 to 2.4 and −2.2 to 1.2 for systolic and diastolic, respectively), standard formula+EBM vs. preterm formula+EBM (95% CI for mean difference in mm Hg: −4.9 to 0.2 and −1.7 to 2.0 for systolic and diastolic, respectively), or exclusive diet of standard formula vs. preterm formula (95% CI for mean difference in mm Hg: −1.6 to 5.8 and −1.6 to 3.9 for systolic and diastolic, respectively).
The investigators conducted two more follow-up studies involving subsets of children from the original cohort. Two hundred and sixteen children (28% of original cohort survivors) were examined at 13–16 years of age.30 Unlike previous studies, the investigators found a modest but significant reduction in the mean [mean difference (95% CI) −4.1 mm Hg (−6.6 to −1.6)] and diastolic [−3.2 mm Hg (−5.8 to −0.6)] blood pressures in children who received exclusive banked DBM (n = 66) vs. those who were assigned to preterm formula (n = 64). These differences remained significant after results were adjusted for sex, sodium intake during infancy, and current body mass index. Interestingly, they found an inverse dose-dependent relation between proportion of enteral intake consumed as human milk during hospital stay and mean [beta (95% CI) = −0.31 mm Hg (−0.52 to −0.10) per 10% increase] and diastolic [beta (95% CI) = −0.28 mm Hg (−0.50 to −0.05)] blood pressures. Although there was no significant difference in birth anthropometric measurements and social class between study population and those who were not followed up, these findings are still limited by a large attrition rate and possible effect of unaccounted confounding variables. The latter is important, as similar studies in term-born individuals have demonstrated that the apparently large difference between groups on univariate analysis can get progressively smaller and eventually disappear as more and more confounding variables get accounted for.34 Nevertheless, this study did highlight the potential long-term cardiovascular-protective effects of human milk-based diet than formula milk, further adding to the growing list of benefits of human milk for premature neonates. Recently, 30 preterm-born adults who were assigned to receive exclusive human milk and 16 preterm-born adults who were assigned to receive an exclusive formula-based diet during hospital stay in the above-mentioned trials underwent detailed cardiovascular assessment between 23 and 28 years of age, including cardiac magnetic resonance imaging.3 There were no significant differences in perinatal characteristics between preterm-born young adults who had been fed exclusively human milk as infants and those exclusively fed formula, including gestational age, days of ventilation, Apgar scores, and exposure to antenatal glucocorticoids. The results were compared with 102 age-matched term-born controls. Expectedly, overall the preterm-born groups demonstrated significant metabolic and cardiac structural differences when compared to the term controls, supporting the known association between prematurity and long-term cardiovascular diseases. These included higher blood glucose and insulin suggesting glucose intolerance and insulin resistance, higher total and low-density lipoprotein cholesterol, and shorter and smaller left and right ventricles, with higher cardiac mass but smaller stroke volumes. However, the reduction in cardiac structural parameters was less profound for the exclusively human milk-fed group in comparison to those who were exclusively formula fed, suggesting a potential protective effect of human milk on long-term cardiac phenotype. Unlike in adolescence, there were no differences seen in either blood biochemistry or any of the blood pressures in relation to the diet in prematurely born groups.
While the data regarding long-term cardio-protective effects of human milk feeding in preterm infants is only at its early stage and acquired from a small number of participants from the same cohort, it appears to be in line with the results of several observational studies conducted in term-born infants.34 This adds to the already known benefits of human milk exposure in these high-risk patients. Moreover, there is no evidence of any long-term deleterious effects of human milk in comparison to formula milk. One of the key missing pieces, however, is the potential mechanism(s) through which human milk feeding may modulate long-term cardiac phenotype in prematurely born infants. One possibility was that this could be an indirect impact of early growth patterns, as infants fed exclusive human milk are known to grow at a slower rate initially than those fed using formula milk. However, studies in preterm infants have failed to find any association between growth patterns in early postnatal period and later cardiovascular outcomes, most notably blood pressure.12 This and the fact that formula milk is often richer in macronutrients indicate that the potential protective impact of human milk may be mediated through other bioactive substances that are not present in formula.
Potential mechanisms of reduced cardiovascular risk
Determining mechanisms to explain why specific factors may improve cardiovascular development is challenging, especially in the preterm infant setting where composition of breast milk varies with gestational age, and the supplementation of mother’s milk with donor milk and fortifiers is common in order to provide sufficient caloric intake. Nevertheless, a number of pathways unique to human milk feeding are likely to contribute to immediate and long-term cardiovascular benefits for the preterm infant, which will be briefly discussed herein.
Growth Factors and Hormones
While breast milk contains a number of different growth factors and hormones involved in organ functional and structural development, vascular endothelial growth factor (VEGF) and adiponectin may be particularly important for the cardiovascular system. VEGF is a key modulator of vasculo-genesis and angiogenesis35 and is most highly expressed in colostrum of both term and preterm human milk.36 Altered vasculo-genesis and angiogenesis in individuals born preterm has been shown as early as birth and extends into adulthood,37,38,39 with a positive relationship with the known increased blood pressure in young adults born preterm.38 Furthermore, dysfunctional VEGF is a potential causal pathway leading to retinopathy of prematurity (ROP).40 Vascularization of the retina in ROP is also affected by immature development of the pulmonary system and hyperoxia induced by supplemental oxygen exposure. Interestingly, VEGF gene therapy has shown to reverse the negative effects of supplemental oxygen and promote lung angiogenesis in a neonatal rat model mimicking BPD conditions.41 The pivotal role of VEGF in alveolarization may be particularly important to understanding the increased pulmonary vascular resistance and risk of pulmonary hypertension in preterm-born young adults, which relate to reductions in right ventricular (RV) function.42 In support of this hypothesis, exclusive human milk feeding during the neonatal period has been shown to relate to greater RV function in young adults born preterm,3 which also related to pulmonary artery dimensions.
Adiponectin is present in large quantities in human milk, is able to pass the intestinal barrier, and has long been studied for its role in cardiovascular diseases, due to its pleiotropic roles in regulating metabolic and inflammatory pathways.17 In addition, adiponectin has been shown to have direct effects on both the vasculature and the heart.43 For instance, adiponectin increases endothelial nitric oxide (NO) synthase activity via 5’ adenosine monophosphate-activated protein kinase, resulting in increased NO production by the endothelium to promote vasodilation. The importance of NO-mediated endothelial vasodilation in cardiovascular diseases, including the pathophysiological development of hypertension, is well studied.44 Adiponectin has also been shown to reduce endothelial oxidative stress and increase endothelial progenitor cell (EPC) mobilization and function while decreasing senescence. Given the known alterations in EPC proliferation and function throughout the life of preterm-born offspring,37,39 intake of adequate levels of dietary adiponectin during the critical early cardiovascular developmental window may be critical. Specific cardioprotective effects of adiponectin may also be relevant to the developing preterm heart, including its ability to suppress apoptosis, oxidative stress, and inflammation in cardiomyocytes.
Immunological and inflammatory factors
A number of different immunoglobulins (secretory IgA, IgG, IgM), cytokines, and chemokines are present in human milk that are involved in prevention of infections, building the infant’s immune system and managing appropriate inflammatory responses. Lactoferrin is also a component of the innate immune system found primarily in mucosa, and provides antibacterial activity in infants. It is present in largest quantities in human colostrum but is expressed in mother’s milk throughout lactation. Lactoferrin is particularly interesting as a target for further research given its anti-inflammatory properties and high expression of the lactoferrin receptor throughout tissues in the body, including the heart and vascular system.45 In addition to the passing of immune-protective factors, a number of different types of stem cells are also uniquely found in human milk, closely resembling human embryonic stem cells and other types of pluripotent stem cells. Remarkably, breast milk stem cells have been shown to differentiate into multiple cell types, including cardiomyocytes.46 Furthermore, these stem cells integrate into different tissues in the infant,47 which suggests that they play a role in normal growth and organ development.
Human milk oligosaccharides
HMOs are a unique type of glycans present in human milk. Concentrations are higher in milk of mothers who delivered preterm, and although >100 different HMOs have now been identified, the synthesis of these different types of HMOs varies greatly between women. Regardless, levels remain 100–1000-fold higher in human milk than from farm animal-derived milk products, such as cow’s and goat’s milk, with a greater diversity of oligosaccharides as well as a higher abundance of fucosylated and lower abundance of sialylated oligosaccharides.48 Though HMOs have been shown to be absorbed by infants and play important roles in immune and inflammatory modulation, it remains to be determined whether HMOs are specifically important for early vascular and cardiac development. Recent studies in animals have shown that HMOs are specifically involved in promoting NO-mediated vasodilation, which may contribute to their role in preventing necrotizing enterocolitis.49 Promotion of NO-mediated vasodilation may therefore also contribute to maintenance of infant hemodynamics and promote normal development of the heart and vascular system.
Shared maternal–offspring benefits
There is growing evidence that breastfeeding is associated with maternal cardiovascular health benefits.50 It is possible that potential mechanisms which ensure cardiovascular wellbeing in mothers who breast feed overlap with those that promote cardiovascular development in the preterm infants who received breast milk feedings. There even may be a dose–response relationship, with positive effects on infant metabolic health, incorporating lipid homeostasis and glucose metabolism, but further research is needed to test these hypotheses.50
Future directions to study the impact of preterm breast milk administration on long-term cardiovascular adaptation
Future studies will need not only to examine preterm myocardial development but also include well-characterized perinatal data, maternal data, and comprehensive plans for prospective follow-up of the infants (and their mothers) from birth through infancy childhood and into adulthood. Long-term maternal–infant cohort studies offer key opportunities to capture the important influence of preconception and obstetric risk factors on cardiovascular health, development, and disease in preterm individuals. The current evidence that comes from observational studies highlights the strong link between early breast milk administrations and improvement in long-term cardiovascular but lacks concrete mechanistic explanations. An in-depth exploration into the composition of breast milk with continued basic science, translational, and preclinical studies to provide mechanisms to explain causality using new methods cannot be overemphasized. Multidisciplinary approaches involving obstetricians, neonatologists, dieticians, cardiologists, primary care providers, lactation consultants, and basic scientists must be encouraged to design and conduct meaningful hypothesis-driven research to study the impact of breast milk administration on the early stages of normal and abnormal cardiovascular growth.
Conclusions
It is becoming increasingly clear that premature birth results in long-term adverse cardiovascular effects with important clinical consequences. There is a distinct lack of prophylactic and therapeutic interventions available to alleviate those effects. Human milk appears to be a vital intervention during a preterm infant’s early life with long-lasting benefits in terms of cardiovascular health and the potential for a significant improvement in the overall quality of life for those infants. Understating the underlying mechanisms and identifying the key components within breast milk that result in improved cardiovascular health in the context of premature birth can pave the way for a more targeted approach for mitigating the effects of preterm birth on long-term cardiovascular wellbeing.
References
Lewandowski, A. J. et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 127, 197–206 (2013).
Lewandowski, A. J. et al. Right ventricular systolic dysfunction in young adults born preterm. Circulation 128, 713–720 (2013).
Lewandowski, A. J. et al. Breast milk consumption in preterm neonates and cardiac shape in adulthood. Pediatrics 138, e20160050 (2016).
Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 129, e827–e841 (2012).
Meier, P. P., Engstrom, J. L., Patel, A. L., Jegier, B. J. & Bruns, N. E. Improving the use of human milk during and after the NICU stay. Clin. Perinatol. 37, 217–245 (2010).
Spiegler, J. et al. Does breastmilk influence the development of bronchopulmonary dysplasia? J. Pediatr. 169, 76.e4–80.e4 (2016).
Zhou, J., Shukla, V. V., John, D. & Chen, C. Human milk feeding as a protective factor for retinopathy of prematurity: a meta-analysis. Pediatrics 136, e1576–e1586 (2015).
Koo, W., Tank, S., Martin, S. & Shi, R. Human milk and neurodevelopment in children with very low birth weight: a systematic review. Nutr. J. 13, 94 (2014).
Horta, B. L., Loret de Mola, C. & Victora, C. G. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. Suppl. 104, 30–37 (2015).
Fewtrell, M. S. Breast-feeding and later risk of CVD and obesity: evidence from randomised trials. Proc. Nutr. Soc. 70, 472–477 (2011).
Behairy, O. G., Fadl, A. M. A., Arafa, O. S., Fadl, A. A. & Attia, M. A. Influence of early feeding practices on biomarkers of cardiovascular disease risk in later life. Egyptian Pediatr. Assoc. Gazette 65, 114–121 (2017).
Lapillonne, A. & Griffin, I. J. Feeding preterm infants today for later metabolic and cardiovascular outcomes. J. Pediatr. 162, S7–S16 (2013).
Rodriguez, N. A. & Caplan, M. S. Oropharyngeal administration of mother’s milk to prevent necrotizing enterocolitis in extremely low-birth-weight infants: theoretical perspectives. J. Perinat. Neonatal Nurs. 29, 81–90 (2015).
Castellote, C. et al. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J. Nutr. 141, 1181–1187 (2011).
Snyder, R. et al. Early provision of oropharyngeal colostrum leads to sustained breast milk feedings in preterm infants. Pediatr. Neonatol. 58, 534–540 (2017).
Sohn, K., Kalanetra, K. M., Mills, D. A. & Underwood, M. A. Buccal administration of human colostrum: impact on the oral microbiota of premature infants. J. Perinatol. 36, 106–111 (2015).
Ballard, O. & Morrow, A. L. Human milk composition: nutrients and bioactive factors. Pediatr. Clin. North Am. 60, 49–74 (2013).
Higgins, R. D. et al. Executive summary of the workshop “Nutritional Challenges in the High Risk Infant”. J. Pediatr. 160, 511–516 (2012).
Gabrielli, O. et al. Preterm milk oligosaccharides during the first month of lactation. Pediatrics 128, e1520–e1531 (2011).
Underwood, M. A. Human milk for the premature infant. Pediatr. Clin. North Am. 60, 189–207 (2013).
Bertino, E. et al. Benefits of human milk in preterm infant feeding. J. Pediatr. Neonatal Indiv. Med. 1, 19–24 (2012).
Coppa, G. V., et al. Glycosaminoglycan content in term and preterm milk during the first month of lactation. Neonatology 101, 74–76 (2012).
Valentine, C. J. & Wagner, C. L. Nutritional management of the breastfeeding dyad. Pediatr. Clin. North Am. 60, 261–274 (2013).
Alsaweed, M., Lai, C., Hartmann, P., Geddes, D. & Kakulas, F. Human milk cells contain numerous miRNAs that may change with milk removal and regulate multiple physiological processes. Int. J. Mol. Sci. 17, 956 (2016).
Bronze-da-Rocha, E. MicroRNAs expression profiles in cardiovascular diseases. Biomed. Res. Int. 2014, 985408 (2014).
Raju, T. N. K., Buist, A. S., Blaisdell, C. J., Moxey-Mims, M. & Saigal, S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr. 106, 1409–1437 (2017).
Crump, C. et al. Association of preterm birth with risk of ischemic heart disease in adulthood. JAMA Pediatr. https://doi.org/10.1001/jamapediatrics.2019.1327 (2019).
Lucas, A. & Morley, R. Does early nutrition in infants born before term programme later blood pressure? BMJ 309, 304–308 (1994).
Lucas, A. et al. Early sodium intake and later blood pressure in preterm infants. Arch. Dis. Child. 63, 656–657 (1988).
Singhal, A., Cole, T. J. & Lucas, A. Early nutrition in preterm infants and later blood pressure: two cohorts after randomised trials. Lancet 357, 413–419 (2001).
Singhal, A., Fewtrell, M., Cole, T. J. & Lucas, A. Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. Lancet 361, 1089–1097 (2003).
Singhal, A., Cole, T. J., Fewtrell, M. & Lucas, A. Breastmilk feeding and lipoprotein profile in adolescents born preterm: follow-up of a prospective randomised study. Lancet 363, 1571–1578 (2004).
Lucas, A. et al. Multicentre trial on feeding low birthweight infants: effects of diet on early growth. Arch. Dis. Child. 59, 722–730 (1984).
Owen, C. G., Whincup, P. H. & Cook, D. G. Breast-feeding and cardiovascular risk factors and outcomes in later life: evidence from epidemiological studies. Proc. Nutr. Soc. 70, 478–484 (2011).
Siafakas, C. G., Anatolitou, F., Fusunyan, R. D., Walker, W. A. & Sanderson, I. R. Vascular endothelial growth factor (VEGF) is present in human breast milk and its receptor is present on intestinal epithelial cells. Pediatr. Res. 45, 652–657 (1999).
Loui, A. et al. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor 1 (sFlt-1) levels in early and mature human milk from mothers of preterm versus term infants. J. Hum. Lact. 28, 522–528 (2012).
Ligi, I. et al. A switch toward angiostatic gene expression impairs the angiogenic properties of endothelial progenitor cells in low birth weight preterm infants. Blood 118, 1699 (2011).
Lewandowski Adam, J. et al. Elevated blood pressure in preterm-born offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension 65, 607–614 (2015).
Bertagnolli, M. et al. Endothelial colony-forming cells in young adults born preterm: a novel link between neonatal complications and adult risks for cardiovascular disease. J. Am. Heart Assoc. 7, e009720 (2018).
Reynolds, J. D. The management of retinopathy of prematurity. Paediatr. Drugs 3, 263–272 (2001).
Thébaud, B. et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury. Circulation 112, 2477–2486 (2005).
Goss, K. N. et al. Early pulmonary vascular disease in young adults born preterm. Am. J. Respir. Crit. Care Med. 198, 1549–1558 (2018).
Hui, X., Lam, K. S. L., Vanhoutte, P. M. & Xu, A. Adiponectin and cardiovascular health: an update. Br. J. Pharmacol. 165, 574–590 (2012).
Farah, C., Michel, L. Y. M. & Balligand, J.-L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 15, 292 (2018).
Suzuki, Y. A., Lopez, V. & Lönnerdal, B. Lactoferrin. Cell. Mol. Life Sci. 62, 2560 (2005).
Hassiotou, F. & Hartmann, P. E. At the dawn of a new discovery: the potential of breast milk stem cells. Adv. Nutr. 5, 770–778 (2014).
Hassiotou, F. et al. Breastmilk stem cell transfer from mother to neonatal organs. FASEB J. 28, 216.4 (2014).
Bode, L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22, 1147–1162 (2012).
Varadharaj, S. et al. The human milk oligosaccharide 3–fucosyllactose facilitates preservation of nitric oxide-induced vasodilation in aortic vessels in vitro. FASEB J. 31, lb808 (2017).
Nguyen, B., Jin, K. & Ding, D. Breastfeeding and maternal cardiovascular risk factors and outcomes: a systematic review. PLoS ONE 12, e0187923 (2017).
Author information
Authors and Affiliations
Contributions
All authors drafted the first manuscript. A.E.-K. developed the concept and P.T.L. provided the initial outline. All authors revised the first draft. Each author named in the manuscript has seen and approved the submission of the version of the manuscript and takes full responsibility. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
EL-Khuffash, A., Jain, A., Lewandowski, A.J. et al. Preventing disease in the 21st century: early breast milk exposure and later cardiovascular health in premature infants. Pediatr Res 87, 385–390 (2020). https://doi.org/10.1038/s41390-019-0648-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0648-5
This article is cited by
-
Arterial hypertension in infants with congenital diaphragmatic hernia following surgical repair
European Journal of Pediatrics (2024)
-
A Mediterranean diet is associated with improved total antioxidant content of human breast milk and infant urine
Nutrition Journal (2023)
-
The association of maternal food quality score (FQS) with breast milk nutrient content and antioxidant content of infant urine: a cross-sectional study
BMC Pregnancy and Childbirth (2023)
-
Maternal Adherence to a Dietary Approaches to Stop Hypertension (DASH) Dietary Pattern and the Relationship to Breast Milk Nutrient Content
Maternal and Child Health Journal (2023)
-
Interactions among maternal smoking, breastfeeding, and offspring genetic factors on the risk of adult-onset hypertension
BMC Medicine (2022)