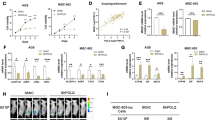
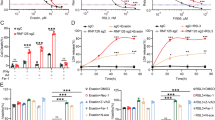
Abstract
Pancreatic cancer cells undergo intricate metabolic reprogramming to sustain their survival and proliferation. p53 exhibits a dual role in tumor cell ferroptosis. However, the precise role and mechanisms underlying wild-type p53 activation in promoting ferroptosis in pancreatic cancer cells remain obscure. In this study, we applied bioinformatics tools and performed an analysis of clinical tissue sample databases and observed a significantly upregulated expression of solute carrier family 35 member F2 (SLC35F2) in pancreatic cancer tissues. Our clinical investigations indicated that elevated SLC35F expression was related to adverse survival outcomes. Through multi-omics analyses, we discerned that SLC35F2 influences the transcriptome and inhibits ferroptosis in pancreatic cancer cells. Moreover, our findings reveal the pivotal involvement of p53 in mediating SLC35F2-mediated ferroptosis, both in vitro and in vivo. SLC35F2 inhibits ferroptosis by facilitating TRIM59-mediated p53 degradation. Further mechanistic investigations demonstrated that SLC35F2 competitively interacts with the E3 ubiquitin ligase SYVN1 of TRIM59, thereby stabilizing TRIM59 expression and consequentially promoting p53 degradation. Utilizing protein 3D structure analysis and drug screening, we identified irinotecan hydrochloride and lapatinib ditosylate as compounds targeting SLC35F2, augmenting the antitumor effect of imidazole ketone erastin (IKE) in a wild-type p53 patient-derived xenograft (PDX) model. However, in the p53 mutant PDX model, irinotecan hydrochloride and lapatinib ditosylate did not alter the sensitivity of the tumor xenograft model to IKE-triggered ferroptosis. In summary, our work establishes a novel mechanism wherein the SLC35F2–SYVN1–TRIM59 axis critically regulates ferroptosis of pancreatic cancer cells by inhibiting endogenous p53. Thus, SLC35F2 emerges as a promising therapeutic target for treating pancreatic cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data in our study are available from the corresponding author upon reasonable request.
References
Park W, Chawla A, O’Reilly EM. Pancreatic cancer: a review. JAMA. 2021;326:851–62.
Zhu H, Li T, Du Y, Li M. Pancreatic cancer: challenges and opportunities. BMC Med. 2018;16:214.
Shin S, Solorzano J, Liauzun M, Pyronnet S, Bousquet C, Martineau Y. Translational alterations in pancreatic cancer: a central role for the integrated stress response. NAR Cancer. 2022;4:zcac031.
Yang J, Jin L, Kim HS, Tian F, Yi Z, Bedi K, et al. KDM6A loss recruits tumor-associated neutrophils and promotes neutrophil extracellular trap formation in pancreatic cancer. Cancer Res. 2022;82:4247–60.
Pirhonen J, Szkalisity A, Hagstrom J, Kim Y, Migh E, Kovacs M, et al. Lipid metabolic reprogramming extends beyond histologic tumor demarcations in operable human pancreatic cancer. Cancer Res. 2022;82:3932–49.
Liu J, Wang Y, Mu C, Li M, Li K, Li S, et al. Pancreatic tumor eradication via selective Pin1 inhibition in cancer-associated fibroblasts and T lymphocytes engagement. Nat Commun. 2022;13:4308.
Chen Y, Yang S, Tavormina J, Tampe D, Zeisberg M, Wang H, et al. Oncogenic collagen I homotrimers from cancer cells bind to alpha3beta1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell. 2022;40:818–34.e819.
Qin C, Yang G, Yang J, Ren B, Wang H, Chen G, et al. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer. 2020;19:50.
Li D, Wang Y, Dong C, Chen T, Dong A, Ren J, et al. CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1. Oncogene. 2023;42:83–98.
Wang CK, Chen TJ, Tan GYT, Chang FP, Sridharan S, Yu CA, et al. MEX3A mediates p53 degradation to suppress ferroptosis and facilitate ovarian cancer tumorigenesis. Cancer Res. 2023;83:251–263.
Chen M, Tan AH, Li J. Curcumin represses colorectal cancer cell proliferation by triggering ferroptosis via PI3K/Akt/mTOR signaling. Nutr Cancer. 2023;75:726–733.
Li J, Xu L, Zuo YX, Chang XQ, Chi HT. Potential intervention target of atherosclerosis: ferroptosis (Review). Mol Med Rep. 2022;26:343.
Pancewicz J, Niklinska WE, Chlanda A. Flake graphene-based nanomaterial approach for triggering a ferroptosis as an attractive theranostic outlook for tackling non-small lung cancer: a mini review. Materials (Basel). 2022;15:3456.
Xu L, Huang X, Lou Y, Xie W, Zhao H. Regulation of apoptosis, autophagy and ferroptosis by non-coding RNAs in metastatic non-small cell lung cancer (Review). Exp Ther Med. 2022;23:352.
Liu M, Kong XY, Yao Y, Wang XA, Yang W, Wu H, et al. The critical role and molecular mechanisms of ferroptosis in antioxidant systems: a narrative review. Ann Transl Med. 2022;10:368.
Moon SH, Huang CH, Houlihan SL, Regunath K, Freed-Pastor WA, Morris JP, et al. p53 represses the mevalonate pathway to mediate tumor suppression. Cell. 2019;176:564–80.e519.
Boutelle AM, Attardi LD. p53 and tumor suppression: it takes a network. Trends Cell Biol. 2021;31:298–310.
Aubrey BJ, Strasser A, Kelly GL. Tumor-suppressor functions of the TP53 pathway. Cold Spring Harb Perspect Med. 2016;6:a026062.
Mantovani F, Collavin L, Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212.
Liu J, Zhang C, Wang J, Hu W, Feng Z. The regulation of ferroptosis by tumor suppressor p53 and its pathway. Int J Mol Sci. 2020;21:8387.
Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162–8.
Kanapathipillai M. Treating p53 mutant aggregation-associated cancer. Cancers (Basel). 2018;10:154.
Nyquist MD, Prasad B, Mostaghel EA. Harnessing solute carrier transporters for precision oncology. Molecules. 2017;22:539.
Hadley B, Litfin T, Day CJ, Haselhorst T, Zhou Y, Tiralongo J. Nucleotide sugar transporter SLC35 family structure and function. Comput Struct Biotechnol J. 2019;17:1123–34.
Bu L, Jiang G, Yang F, Liu J, Wang J. Highly expressed SLC35F2 in non-small cell lung cancer is associated with pathological staging. Mol Med Rep. 2011;4:1289–93.
Kotolloshi R, Holzer M, Gajda M, Grimm MO, Steinbach D. SLC35F2, a transporter sporadically mutated in the untranslated region, promotes growth, migration, and invasion of bladder cancer cells. Cells. 2021;10:80.
Chiou JT, Lee YC, Huang CH, Wang LJ, Shi YJ, Chang LS. Inhibition of Sp1-mediated survivin and MCL1 expression cooperates with SLC35F2 and myeloperoxidase to modulate YM155 cytotoxicity to human leukemia cells. Biochem Pharm. 2021;188:114544.
Winter GE, Radic B, Mayor-Ruiz C, Blomen VA, Trefzer C, Kandasamy RK, et al. The solute carrier SLC35F2 enables YM155-mediated DNA damage toxicity. Nat Chem Biol. 2014;10:768–73.
Luo Y, Gao X, Zou L, Lei M, Feng J, Hu Z. Bavachin induces ferroptosis through the STAT3/P53/SLC7A11 axis in osteosarcoma cells. Oxid Med Cell Longev. 2021;2021:1783485.
Ma S, Sun L, Wu W, Wu J, Sun Z, Ren J. USP22 protects against myocardial ischemia-reperfusion injury via the SIRT1-p53/SLC7A11-dependent inhibition of ferroptosis-induced cardiomyocyte death. Front Physiol. 2020;11:551318.
Guan Z, Chen J, Li X, Dong N. Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation. Biosci Rep. 2020;40:BSR20201807.
Engeland K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022;29:946–60.
Rocha S, Campbell KJ, Roche KC, Perkins ND. The p53-inhibitor pifithrin-alpha inhibits firefly luciferase activity in vivo and in vitro. BMC Mol Biol. 2003;4:9.
Zhou Z, Ji Z, Wang Y, Li J, Cao H, Zhu HH, et al. TRIM59 is up-regulated in gastric tumors, promoting ubiquitination and degradation of p53. Gastroenterology. 2014;147:1043–54.
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64.
Sha W, Hu F, Xi Y, Chu Y, Bu S. Mechanism of ferroptosis and its role in type 2 diabetes mellitus. J Diabetes Res. 2021;2021:9999612.
Wang Y, Wei Z, Pan K, Li J, Chen Q. The function and mechanism of ferroptosis in cancer. Apoptosis. 2020;25:786–98.
Zhu J, Xiong Y, Zhang Y, Wen J, Cai N, Cheng K, et al. The molecular mechanisms of regulating oxidative stress-induced ferroptosis and therapeutic strategy in tumors. Oxid Med Cell Longev. 2020;2020:8810785.
Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843.
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85.
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12:34.
Zhang S, Li Q, Yuan H, Ren L, Liang X, Li S, et al. Solute carrier family 35 member F2 regulates cisplatin resistance and promotes malignant progression of pancreatic cancer by regulating RNA binding motif protein 14. J Oncol. 2022;2022:5091154.
Ayka A, Sehirli AO. The role of the SLC transporters protein in the neurodegenerative disorders. Clin Psychopharmacol Neurosci. 2020;18:174–87.
Gupta S, Burckhardt G, Hagos Y. SLC22 transporter family proteins as targets for cytostatic uptake into tumor cells. Biol Chem. 2011;392:117–24.
Nishimura M, Suzuki S, Satoh T, Naito S. Tissue-specific mRNA expression profiles of human solute carrier 35 transporters. Drug Metab Pharmacokinet. 2009;24:91–99.
Liu Y, Gu W. p53 in ferroptosis regulation: the new weapon for the old guardian. Cell Death Differ. 2022;29:895–910.
Chen LL, Wang WJ. p53 regulates lipid metabolism in cancer. Int J Biol Macromol. 2021;192:45–54.
Yu L, Wu M, Zhu G, Xu Y. Emerging roles of the tumor suppressor p53 in metabolism. Front Cell Dev Biol. 2021;9:762742.
Mao C, Wang X, Liu Y, Wang M, Yan B, Jiang Y, et al. A G3BP1-interacting lncrna promotes ferroptosis and apoptosis in cancer via nuclear sequestration of p53. Cancer Res. 2018;78:3484–96.
Pan M, Blattner C. Regulation of p53 by E3s. Cancers (Basel). 2021;13:745.
Liu R, Li H, Xu Y, Li X, Guo X, Shi J, et al. Blockade of TRIM59 enhances esophageal cancer cell chemosensitivity to cisplatin by upregulating p53. Oncol Lett. 2021;21:6.
Chen S, Du Y, Xu B, Li Q, Yang L, Jiang Z, et al. Vaccinia-related kinase 2 blunts sorafenib’s efficacy against hepatocellular carcinoma by disturbing the apoptosis-autophagy balance. Oncogene. 2021;40:3378–93.
Chen L, Yuan R, Wen C, Liu T, Feng Q, Deng X, et al. E3 ubiquitin ligase UBR5 promotes pancreatic cancer growth and aerobic glycolysis by downregulating FBP1 via destabilization of C/EBPalpha. Oncogene. 2021;40:262–76.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82203493,82372769, 82060454, 81960522, 82160364, 82060449, and 82060466), the Project of the Jiangxi Provincial Department of Science and Technolog (20212ACB206024, 20212BCJ23022, 20232BCJ22016, 20224BAB206081, 20202BABL206084, 20192BAB205068, 20212BAB206044, and 20202BAB216032). The key research and development program of Jiangxi Province of China (20203BBGL73143). Outstanding Youth Fund of Jiangxi Cancer Hospital (2021DYS06).
Author information
Authors and Affiliations
Contributions
FLC conceived the research concept and design. FLC, KW, MW, WZ, and XXL implemented the methodological development and drafted, reviewed, and revised the manuscript. BC, YYD, FRY, HX, JS, SHZ, and QF collected, analyzed, and interpretated the data and conducted statistical analysis of the data; MFX, YY, LH, and XHD extended material and technical support. All the authors read and authorized the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Che, B., Du, Y., Yuan, R. et al. SLC35F2–SYVN1–TRIM59 axis critically regulates ferroptosis of pancreatic cancer cells by inhibiting endogenous p53. Oncogene 42, 3260–3273 (2023). https://doi.org/10.1038/s41388-023-02843-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02843-y