Abstract
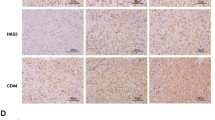
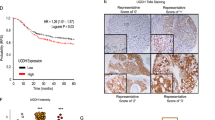
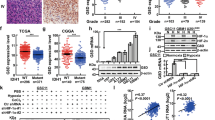
UDP-glucose 6-dehydrogenase (UGDH) produces UDP-α-d-glucuronic acid, the precursors for glycosaminoglycans (GAGs) and proteoglycans of the extracellular matrix. Elevated GAG formation has been implicated in a variety of human diseases, including glioblastoma (GBM). In our previous study, we found that Krüppel-like factor 4 (KLF4) promotes GBM cell migration by binding to methylated DNA, mainly methylated CpGs (mCpG) and transactivating gene expression. We identified UDGH as one of the downstream targets of KLF4–mCpG binding activity. In this study, we show that KLF4 upregulates UGDH expression in a mCpG-dependent manner, and UGDH is required for KLF4-induced cell migration in vitro. UGDH knockdown decreases GAG abundance in GBM cells, as well as cell proliferation and migration in vitro. In intracranial xenografts, reduced UGDH inhibits tumor growth and migration, accompanied by a decrease in the expression of extracellular matrix proteins such as tenascin C, brevican. Our studies demonstrate a novel DNA methylation-dependent UGDH upregulation by KLF4. Developing UGDH antagonists to decrease the synthesis of extracellular matrix components will be a useful strategy for GBM therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–83.
Batash R, Asna N, Schaffer P, Francis N, Schaffer M. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr Med Chem. 2017;24:3002–9.
Marzenna W, Rojiani MV. Extracellular matrix microenvironment in glioma progression. In: Ghosh A, editor. Glioma - Exploring Its Biology and Practical Relevance. InTech; 2011;(Chap 12):257–284.
Venning FA, Wullkopf L, Erler JT. Targeting ECM disrupts cancer progression. Front Oncol. 2015;5:224.
Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406.
Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karousou E, Theocharis AD, et al. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 2012;279:1177–97.
Wan J, Su Y, Song Q, Tung B, Oyinlade O, Liu S, et al. Methylated cis-regulatory elements mediate KLF4-dependent gene transactivation and cell migration. eLife. 2017;6:e20068.
Hu S, Wan J, Su Y, Song Q, Zeng Y, Nguyen HN, et al. DNA methylation presents distinct binding sites for human transcription factors. eLife. 2013;2:e00726.
Egger S, Chaikuad A, Kavanagh KL, Oppermann U, Nidetzky B. UDP-glucose dehydrogenase: structure and function of a potential drug target. Biochem Soc Trans. 2010;38:1378–85.
Clarkin CE, Allen S, Kuiper NJ, Wheeler BT, Wheeler-Jones CP, Pitsillides AA. Regulation of UDP-glucose dehydrogenase is sufficient to modulate hyaluronan production and release, control sulfated GAG synthesis, and promote chondrogenesis. J Cell Physiol. 2011;226:749–61.
Wen Y, Li J, Wang L, Tie K, Magdalou J, Chen L, et al. UDP-glucose dehydrogenase modulates proteoglycan synthesis in articular chondrocytes: its possible involvement and regulation in osteoarthritis. Arthritis Res Ther. 2014;16:484.
Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39.
Park JB, Kwak H-J, Lee S-H. Role of hyaluronan in glioma invasion. Cell Adhes Migr. 2008;2:202–7.
Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058
Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21.
Yu F, Li J, Chen H, Fu J, Ray S, Huang S, et al. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011;30:2161–72.
Tilghman J, Wu H, Sang Y, Shi X, Guerrero-Cazares H, Quinones-Hinojosa A, et al. HMMR maintains the stemness and tumorigenicity of glioblastoma stem-like cells. Cancer Res. 2014;74:3168–79.
Ying M, Tilghman J, Wei Y, Guerrero-Cazares H, Quinones-Hinojosa A, Ji H, et al. Kruppel-like factor-9 (KLF9) inhibits glioblastoma stemness through global transcription repression and integrin alpha6 inhibition. J Biol Chem. 2014;289:32742–56.
Wu Y, Richard JP, Wang SD, Rath P, Laterra J, Xia S. Regulation of glioblastoma multiforme stem-like cells by inhibitor of DNA binding proteins and oligodendroglial lineage-associated transcription factors. Cancer Sci. 2012;103:1028–37.
Rath P, Lal B, Ajala O, Li Y, Xia S, Kim J, et al. In vivo c-Met pathway inhibition depletes human glioma xenografts of tumor-propagating stem-like cells. Transl Oncol. 2013;6:104–11.
Xia S, Lal B, Tung B, Wang S, Goodwin CR, Laterra J. Tumor microenvironment tenascin-C promotes glioblastoma invasion and negatively regulates tumor proliferation. Neuro-Oncology. 2016;18:507–17.
Barbosa I, Garcia S, Barbier-Chassefière V, Caruelle J-P, Martelly I, Papy-García D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology. 2003;13:647–53.
Wang SD, Rath P, Lal B, Richard JP, Li Y, Goodwin CR, et al. EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene. 2012;31:5132–43.
Reznik TE, Sang Y, Ma Y, Abounader R, Rosen EM, Xia S, et al. Transcription-dependent epidermal growth factor receptor activation by hepatocyte growth factor. Mol Cancer Res. 2008;6:139–50.
Wade A, Robinson AE, Engler JR, Petritsch C, James CD, Phillips JJ. Proteoglycans and their roles in brain cancer. FEBS J. 2013;280:2399–417.
Nutt CL, Zerillo CA, Kelly GM, Hockfield S. Brain enriched hyaluronan binding (BEHAB)/brevican increases aggressiveness of CNS-1 gliomas in Lewis rats. Cancer Res. 2001;61:7056–9.
Craig JM, Bickmore WA. The distribution of CpG islands in mammalian chromosomes. Nat Genet. 1994;7:376–82.
Medvedeva YA, Khamis AM, Kulakovskiy IV, Ba-Alawi W, Bhuyan MSI, Kawaji H, et al. Effects of cytosine methylation on transcription factor binding sites. BMC Genom. 2014;15:119.
Acknowledgements
We thank Dr. H Lopez-Bertoni for technical expertise.
Fundng
This work was supported by grants from NIH R01NS091165 (to SX), NIH R01 NS096754 (to JL), NS076759 (to JL), NIH GM111514 (to HZ), NIH R01 GM111514 (to HZ), NIH R33CA186790 (to HZ), NIH U54 HG006434 (to HZ), NIH U24 CA160036 (to HZ), Ford Foundation pre-doctoral fellowship program (to OO) and NIH T32 GM007445 (to OO), NIH/NINDS K12 Physician Scientist Award, Burroughs Wellcome Fund, and Duke SPORE CEP Award (to CRG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Oyinlade, O., Wei, S., Lal, B. et al. Targeting UDP-α-d-glucose 6-dehydrogenase inhibits glioblastoma growth and migration. Oncogene 37, 2615–2629 (2018). https://doi.org/10.1038/s41388-018-0138-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0138-y
This article is cited by
-
UDP-glucuronate metabolism controls RIPK1-driven liver damage in nonalcoholic steatohepatitis
Nature Communications (2023)
-
UGDH promotes tumor-initiating cells and a fibroinflammatory tumor microenvironment in ovarian cancer
Journal of Experimental & Clinical Cancer Research (2023)
-
Spatiotemporal dissection of the cell cycle with single-cell proteogenomics
Nature (2021)
-
Chondroitin sulfate synthase 1 enhances proliferation of glioblastoma by modulating PDGFRA stability
Oncogenesis (2020)
-
UDP-glucose 6-dehydrogenase regulates hyaluronic acid production and promotes breast cancer progression
Oncogene (2020)