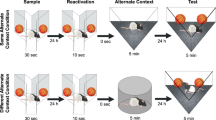
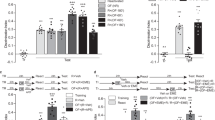
Abstract
Long-term memory storage is a dynamic process requiring flexibility to ensure adaptive behavioural responding in changing environments. Indeed, it is well established that memory reactivation can “destabilize” consolidated traces, leading to various forms of updating. However, the neurobiological mechanisms rendering long-term memories labile and modifiable remain poorly described. Moreover, boundary conditions, such as the age or strength of the memory, can reduce the likelihood of this destabilization; yet, intuitively, these most behaviourally influential of memories should also be modifiable under appropriate conditions. Here, we provide evidence that salient novelty at the time of memory reactivation promotes integrative updating of resistant object memories in rats. Furthermore, blockade of muscarinic acetylcholine receptors (mAChRs; with pirenzepine) or disruption of calcium/calmodulin (Ca2+/CaM) with KN-93, a Ca2+/CaM-binding molecule that inhibits calcium/calmodulin-dependent protein kinase II (CaMKII) activation, in perirhinal cortex (PRh) prevented novelty-induced destabilization and updating of resistant object memories. Finally, PRh M1 mAChR activation (with CDD-0102A) was sufficient to destabilize resistant object memories for updating, and this effect was blocked by KN-93, possibly via inhibition of CaMKII activity. Thus, mAChRs and activation of CaMKII appear to interact as part of a mechanism to override boundary conditions on resistant object memories to ensure integrative modification with novel information. These findings therefore have important implications for understanding the dynamic nature of long-term memory storage and potential treatments for conditions characterized by maladaptive and inflexible memories.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nader K, Schafe G, LeDoux J. The labile nature of consolidation theory. Nat Rev. 2000;1:216–9.
Nader K. Reconsolidation and the dynamic nature of memory. Cold Spring Harb Perspect Biol. 2015;7:1–18.
Finnie PSB, Nader K. The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation. Neurosci Biobehav Rev. 2012;36:1667–707.
Lee JL, Nader K, Schiller D. An update on memory reconsolidation updating. Trends Cogn Sci. 2017;21:531–45.
Jardine KH, Huff AE, Wideman CE, McGraw SD, Winters BD. The evidence for and against reactivation-induced memory updating in humans and nonhuman animals. Neurosci Biobehav Rev. 2022;136:1–24.
Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–34.
Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86.
Boccia MM, Blake MG, Acosta GB, Baratti CM. Post-retrieval effects of icv infusions of hemicholinium in mice are dependent on the age of the original memory. Learn Mem. 2006;13:376–81.
Einarsson EÖ, Nader K. Involvement of the anterior cingulate cortex in formation, consolidation, and reconsolidation of recent and remote contextual fear memory. Learn Mem. 2012;19:449–52.
Eisenberg M, Dudai Y. Reconsolidation of fresh, remote, and extinguished fear memory in medaka: Old fears don’t die. Eur J Neurosci. 2004;20:3397–403.
Frankland PW, Ding HK, Takahashi E, Suzuki A, Kida S, Silva AJ. Stability of recent and remote contextual fear memory. Learn Mem. 2006;13:451–7.
Litvin OO, Anokhin KV. Mechanisms of memory reorganization during retrieval of acquired behavioral experience in chicks: The effects of protein synthesis inhibition in the brain. Neurosci Behav Physiol. 2000;30:671–8.
Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–5.
Robinson MJF, Franklin KBJ. Reconsolidation of a morphine place preference: Impact of the strength and age of memory on disruption by propranolol and midazolam. Behav Brain Res. 2010;213:201–7.
Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–95.
Winters BD, Tucci MC, DaCosta-Furtado M. Older and stronger object memories are selectively destabilized by reactivation in the presence of new information. Learn Mem. 2009;16:545–53.
Huff AE, McGraw SD, Winters BD. Muscarinic (M1) cholinergic receptor activation within the dorsal hippocampus promotes destabilization of strongly encoded object location memories. Hippocampus. 2022;32:55–66.
Wang SH, De Oliveira Alvares L, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci. 2009;12:905–12.
Wideman CE, Jardine KH, Winters BD. Involvement of classical neurotransmitter systems in memory reconsolidation: Focus on destabilization. Neurobiol Learn Mem. 2018;156:68–79.
Das RK, Gale G, Hennessy V, Kamboj SK. A prediction error-driven retrieval procedure for destabilizing and rewriting maladaptive reward memories in hazardous drinkers. J Vis Exp. 2018;2018:1–12.
Choi J-H, Kim J-E, Kaang B-K. Protein synthesis and degradation are required for the incorporation of modified information into the pre-existing object-location memory. Mol Brain. 2010;3:1.
Díaz-Mataix L, Ruiz Martinez RC, Schafe GE, Ledoux JE, Doyère V. Detection of a temporal error triggers reconsolidation of amygdala-dependent memories. Curr Biol. 2013;23:467–72.
Exton-McGuinness MTJ, Lee JLC, Reichelt AC. Updating memories-The role of prediction errors in memory reconsolidation. Behav Brain Res. 2015;278:375–84.
Fernández RS, Boccia MM, Pedreira ME. The fate of memory: reconsolidation and the case of prediction error. Neurosci Biobehav Rev. 2016;68:423–41.
Jarome TJ, Ferrara NC, Kwapis JL, Helmstetter FJ. Contextual information drives the reconsolidation-dependent updating of retrieved fear memories. Neuropsychopharmacology. 2015;40:3044–52.
Pedreira ME, Pérez-Cuesta LM, Maldonado H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn Mem. 2004;11:579–85.
Reichelt AC, Exton-McGuinness MT, Lee JLC. Ventral tegmental dopamine dysregulation prevents appetitive memory destabilization. J Neurosci. 2013;33:14205–10.
Rossato J, Bevilaqua L, Myskiw J, Medina J, Izquierdo I, Cammarota M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem. 2007;14:36–46.
Gotthard GH, Gura H. Visuospatial word search task only effective at disrupting declarative memory when prediction error is present during retrieval. Neurobiol Learn Mem. 2018;156:80–85.
Junjiao L, Wei C, Jingwen C, Yanjian H, Yong Y, Liang X, et al. Role of prediction error in destabilizing fear memories in retrieval extinction and its neural mechanisms. Cortex. 2019;121:292–307.
Sevenster D, Beckers T, Kindt M. Prediction Error Governs Pharmacologically Induced Amnesia for Learned Fear. Science (80-). 2013;339:830–4.
Simon KCNS, Gómez RL, Nadel L, Scalf PE. Brain correlates of memory reconsolidation: A role for the TPJ. Neurobiol Learn Mem. 2017;142:154–61.
Sinclair AH, Manalili GM, Brunec IK, Adcock RA, Barense MD. Prediction Error Disrupts Hippocampal Representations and Update Episodic Memories. PNAS. 2021;118:1–12.
Sinclair AH, Barense MD. Surprise and destabilize: Prediction error influences episodic memory reconsolidation. Learn Mem. 2018;25:369–81.
Sinclair AH, Barense MD. Prediction error and memory reactivation: how incomplete reminders drive reconsolidation. Trends Neurosci. 2019;42:727–39.
Agustina López M, Jimena Santos M, Cortasa S, Fernández RS, Carbó Tano M, Pedreira ME. Different dimensions of the prediction error as a decisive factor for the triggering of the reconsolidation process. Neurobiol Learn Mem. 2016;136:210–9.
Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience. 2001;106:43–53.
Hasselmo ME, Bower JM. Acetylcholine and memory. Trends Neurosci. 1993;16:218–22.
Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004;145:207–31.
Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Rev. 1997;23:28–46.
Winters BD, Saksida L, Bussey TJ. Paradoxical facilitation of object recognition memory after infusion of scopolamine into perirhinal cortex: implications for cholinergic system function. J Neurosci. 2006;26:9520–9.
Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–29.
Stiver ML, Jacklin DL, Mitchnick KA, Vicic N, Carlin J, O’Hara M, et al. Cholinergic manipulations bidirectionally regulate object memory destabilization. Learn Mem. 2015;22:203–14.
Stiver ML, Cloke JM, Nightingale N, Rizos J, Messer WS, Winters BD. Linking muscarinic receptor activation to UPS-mediated object memory destabilization: Implications for long-term memory modification and storage. 2017;145:151–64.
Lee S-H, Choi J-H, Lee N, Lee H-R, Kim J-I, Yu N-K, et al. Synaptic protein degredation underlies destabilization of retrieved fear memory. Science (80-). 2008;319:1253–6.
Jarome TJ, Ferrara NC, Kwapis JL, Helmstetter FJ. CaMKII regulates proteasome phosphorylation and activity and promotes memory destabilization following retrieval. Neurobiol Learn Mem. 2016;128:103–9.
Jarome TJ, Werner CT, Kwapis JL, Helmstetter FJ. Activity dependent protein degradation is critical for the formation and stability of fear memory in the amygdala. PLoS One. 2011;6:e24349.
Felder CC. Muscarini acetylcholine receptors: signal transduction through multiple effectors. FASEB J. 1995;9:619–25.
Djakovic SN, Schwarz LA, Barylko B, DeMartino GN, Patrick GN. Regulation of the proteasome by neuronal activity and calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2009;284:26655–65.
Jarome TJ, Helmstetter FJ. Protein degradation and protein synthesis in long-term memory formation. Front Mol Neurosci. 2014;7:1–12.
Vigil FA, Giese KP. Calcium/calmodulin-dependent protein kinase II and memory destabilization: a new role in memory maintenance. J Neurochem. 2018;145:0–3.
Jardine KH, Wideman CE, MacGregor C, Sgarbossa C, Orr D, Mitchnick KA, et al. Activation of cortical M1 muscarinic receptors and related intracellular signaling is necessary for reactivation-induced object memory updating. Sci Rep. 2020;10:1–14.
Wideman CE, Nguyen J, Jeffries SD, Winters BD. Fluctuating NMDA receptor subunit levels in perirhinal cortex relate to their dynamic roles in object memory destabilization and reconsolidation. Int J Mol Sci. 2020;22:1–19.
Rich MT, Abbott TB, Chung L, Gulcicek EE, Stone KL, Colangelo CM, et al. Phosphoproteomic analysis reveals a novel mechanism of CaMKIIα regulation inversely induced by cocaine memory extinction versus reconsolidation. J Neurosci. 2016;36:7613–27.
Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–8.
Winters BD, Tucci MC, Jacklin DL, Reid JM, Newsome J. On the dynamic nature of the engram: evidence for circuit-level reorganization of object memory traces following reactivation. J Neurosci. 2011;31:17719–28.
Ennaceur A, DeLacour J. A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory. Behav Brain Res. 1988;51:83–92.
Burwell RD. Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. J Comp Neurol. 2001;437:17–41.
Cao X, Wang H, Mei B, An S, Yin L, Wang LP, et al. Inducible and selective erasure of memories in the mouse brain via chemical-genetic manipulation. Neuron. 2008;60:353–66.
Vigil FA, Mizuno K, Lucchesi W, Valls-Comamala V, Giese KP. Prevention of long-term memory loss after retrieval by an endogenous CaMKII inhibitor. Sci Rep. 2017;7:4040–9.
Wong MH, Samal AB, Lee M, Vlach J, Novikov N, Niedziela-Majka A, et al. The KN-93 molecule inhibits calcium/calmodulin-dependent protein kinase II (CaMKII) activity by binding to Ca2+/CaM. J Mol Biol. 2019;431:1440–59.
Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006;9:1237–9.
Milton AL, Merlo E, Ratano P, Gregory BL, Dumbreck JK, Everitt BJ. Double dissociation of the requirement for GluN2B- and GluN2A-containing NMDA receptors in the destabilization and restabilization of a reconsolidating memory. J Neurosci. 2013;33:1109–15.
Haubrich J, Crestani AP, Cassini LF, Santana F, Sierra RO, De O Alvares L, et al. Reconsolidation allows fear memory to be updated to a less aversive level through the incorporation of appetitive information. Neuropsychopharmacology. 2015;40:315–26.
Ferrer Monti RI, Giachero M, Alfei JM, Bueno AM, Cuadra G, Molina VA. An appetitive experience after fear memory destabilization attenuates fear retention: involvement GluN2B-NMDA receptors in the Basolateral Amygdala Complex. Learn Mem. 2016;23:465–78.
Lee JLC. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci. 2008;11:1264–6.
Wright DS, Bodinayake KK, Kwapis JL. Investigating memory updating in mice using the objects in updated locations task. Curr Protoc Neurosci. 2020;91:e87.
Creighton SD, Mendell AL, Palmer D, Kalisch BE, MacLusky NJ, Prado VF, et al. Dissociable cognitive impairments in two strains of transgenic Alzheimer’s disease mice revealed by a battery of object-based tests. Sci Rep. 2019;9:1–12.
Lee JLC. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–20.
Funding
This research was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to BDW (400176) and NSERC Canadian Graduate Scholarship Doctorial Awards to CEW and AEH.
Author information
Authors and Affiliations
Contributions
CEW and AEH conducted behavioural testing. WSM developed and donated CDD-0102A. CEW and BDW conceptualized and designed experiments, conducted data analyses, and wrote and edited the manuscript. All authors approve of the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wideman, C.E., Huff, A.E., Messer, W.S. et al. Muscarinic receptor activation overrides boundary conditions on memory updating in a calcium/calmodulin-dependent manner. Neuropsychopharmacol. 48, 1358–1366 (2023). https://doi.org/10.1038/s41386-023-01564-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01564-w