Abstract
Current knowledge about functional connectivity in obsessive-compulsive disorder (OCD) is based on small-scale studies, limiting the generalizability of results. Moreover, the majority of studies have focused only on predefined regions or functional networks rather than connectivity throughout the entire brain. Here, we investigated differences in resting-state functional connectivity between OCD patients and healthy controls (HC) using mega-analysis of data from 1024 OCD patients and 1028 HC from 28 independent samples of the ENIGMA-OCD consortium. We assessed group differences in whole-brain functional connectivity at both the regional and network level, and investigated whether functional connectivity could serve as biomarker to identify patient status at the individual level using machine learning analysis. The mega-analyses revealed widespread abnormalities in functional connectivity in OCD, with global hypo-connectivity (Cohen’s d: -0.27 to -0.13) and few hyper-connections, mainly with the thalamus (Cohen’s d: 0.19 to 0.22). Most hypo-connections were located within the sensorimotor network and no fronto-striatal abnormalities were found. Overall, classification performances were poor, with area-under-the-receiver-operating-characteristic curve (AUC) scores ranging between 0.567 and 0.673, with better classification for medicated (AUC = 0.702) than unmedicated (AUC = 0.608) patients versus healthy controls. These findings provide partial support for existing pathophysiological models of OCD and highlight the important role of the sensorimotor network in OCD. However, resting-state connectivity does not so far provide an accurate biomarker for identifying patients at the individual level.
Similar content being viewed by others
Introduction
Obsessive-compulsive disorder (OCD) is a debilitating disorder with an estimated lifetime prevalence of 1–3% worldwide [1]. It is characterized by intrusive, irrational and distressing thoughts (obsessions) and repetitive physical or mental acts (compulsions) [2]. Prevailing models of OCD propose that symptomatology is associated with structural and functional brain abnormalities within cortico-striato-thalamo-cortical (CSTC) circuits related to motor, cognitive, affective, and motivational processes [3,4,5,6,7,8]. These CSTC circuits form parallel, partly segregated feedback loops, projecting from different cortical regions through specific striatal regions to the thalamus with recurrent connections back to the cortex [7,8,9,10,11]. The most recent addition to the CSTC disease model for OCD is the sensorimotor circuit [7, 8]. This circuit includes cortical and subcortical regions involved in the generation and control of motor behaviors and integration of sensory information [7, 8]. The sensorimotor circuit is particularly relevant to OCD given its role in habit formation, sensory motor gating, and inhibitory control processes that could be related to the inability of patients to suppress internally triggered repetitive and intrusive thoughts and behavior [12,13,14,15,16]. It is now recognized that brain regions beyond the CSTC circuitry, including those in frontolimbic, frontoparietal, and cerebellar networks are also involved in OCD [6, 7, 16].
Studies have used resting-state functional connectivity (FC) analyses to investigate the pathophysiology of OCD, which examine the statistical dependencies between the fluctuations in blood oxygenation level–dependent (BOLD) signal of anatomically separated brain regions or networks [17]. Abnormalities in FC have been proposed as candidate markers of psychopathological conditions and as potential predictor of therapeutic outcomes [18, 19]. The majority of OCD resting-state studies have investigated FC in large-scale networks and a priori selected brain regions (seeds) involved in CSTC circuits. A recent meta-analysis evaluated 34 seed-based FC studies in OCD by categorizing seed regions into predefined networks [20]. Results indicated lower connectivity between frontoparietal (FPN; also referred to as “central-executive control”), salience (SN), and default-mode (DMN) networks in line with the proposed “triple network” model of psychopathology [21], as well as altered connectivity (no specific direction of connectivity change) within FPN and striatal regions [20]. Another more recent meta-analysis specifically focused on FC of seed regions that were consistently used across 47 included studies [22]. Here the authors reported altered connectivity between the striatum and cortical networks (caudate hypo-connectivity with FPN regions; caudate hyper-connectivity and nucleus accumbens hypo-connectivity with frontolimbic regions), hypo-connectivity between the striatum and thalamus, and altered connectivity between cingulate and frontolimbic regions (i.e. hyper-connectivity with the ventromedial prefrontal cortex [vmPFC] and hypo-connectivity with the dorsolateral PFC [dlPFC]) [22]. These meta-analyses provide evidence for regions within the CSTC circuitry and large-scale brain networks to play a role in OCD. However, findings are only partially consistent and are primarily based on small, single center studies that do not reflect the wide range of clinical heterogeneity in OCD and may have poor generalizability [23, 24], and have been prone to publication bias [22]. Additionally, the majority of FC studies have tested a limited set of hypotheses and have used seed-based FC of predefined brain regions and appointed networks rather than connectivity throughout the brain.
Against this background, we investigated FC differences across the entire brain using mega-analysis of resting-state functional MRI data of 1024 OCD patients and 1028 healthy controls (HC) from 28 independent samples of the Enhancing Neuro-Imaging and Genetics through Meta-Analysis (ENIGMA) OCD consortium. We assessed group differences in whole-brain FC (i.e., the functional connectome) at both the regional and network level. A whole-brain seed-based approach was chosen to potentially identify altered FC related to OCD in regions and networks that might have been overlooked in previous hypothesis-driven FC studies. Therefore, we did not test specific hypotheses in this study. Recent studies from the ENIGMA-OCD working group have shown distinct alterations in brain structure for different age groups [3, 25]. Pediatric (<18 years) patients showed larger thalamic volume, thinner superior and inferior parietal cortices compared to HC, whereas adult (≥18 years) patients were found to have larger pallidal and smaller hippocampal volumes, lower surface area for the transverse temporal cortex and a thinner inferior parietal cortex [3, 25]. We therefore performed our analyses separately for adult and pediatric participants and also aimed to establish the potential modulating effects of clinical characteristics (i.e., disease severity, age of onset, and medication use) consistent with previous studies from the working group [3, 25, 26]. Additionally, we investigated whether FC could serve as a biomarker to identify patients at the individual level using machine learning analysis [27].
Methods
Study population
Data were provided by the ENIGMA-OCD working group and initially comprised 36 independent samples from 24 research institutes around the globe, with neuroimaging and clinical data from adult (≥18 years) and pediatric (<18 years) samples. We considered data from 2895 participants, including 1495 OCD patients (1279 adult, 216 pediatric) and 1400 HC (free of psychopathology and psychotropic medication; 1220 adult, 180 pediatric). Diagnosis was determined in accordance with DSM-IV(-TR) or DSM-5 criteria using structured interviews (see Supplementary Methods for an overview of the questionnaires used). Illness severity was measured using the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) and the Children’s Y-BOCS [28, 29]. We excluded two HC who were using psychotropic medication, 264 participants whose data failed neuroimaging quality control, 111 participants due to excessive motion, 315 participants with insufficient brain coverage and 151 participants from samples with <10 participants per group (see Supplementary Methods and Supplementary Figure 1 for flowchart), resulting in a final sample of 2052 participants from 28 samples including 1024 OCD patients (912 adult, 112 pediatric) and 1028 HC (923 adult, 105 pediatric). An overview of demographic and clinical characteristics can be found in Supplementary Table S1. All participating sites obtained permission from their local institutional review boards or ethics committees to provide coded, de-identified data for analysis, and all study participants or caregivers provided written informed consent.
Image acquisition and processing
Structural T1-weighted (T1w) and resting-state functional brain MRI data were acquired at 1.5 or 3 tesla and preprocessed locally at each site. rs-fMRI data were obtained for 4–12 min with a repetition time ranging between 700 and 3500 ms (see Supplementary Table S2). The images were analyzed using HALFpipe (Harmonized AnaLysis of Functional MRI pipeline) versions 1.0.0 to 1.2.1 [30], which is based on fMRIPrep [31], following standardized protocols to harmonize analysis and quality control across multiple sites (see http://enigma.ini.usc.edu/protocols/functional-protocols/). Preprocessing included motion correction, slice timing and susceptibility distortion correction (if slice timing details and field maps were available), and spatial normalization. Denoising was performed after resampling the images to standard space and included spatial smoothing, grand mean scaling, and ICA-AROMA to regress out motion artifacts related to head motion, white matter (WM), and cerebrospinal fluid (CSF) [32, 33], and temporal filtering using either band- or high pass filtering (using a Gaussian-weighted high-pass width of 125 s for FC, and a frequency-based band pass filter with a low cut-off of 0.01 Hz and a high cut-off of 0.1 Hz for measures of local brain activity). Additionally, physiological nuisance regressors were extracted for anatomical component correction (aCompCor) using the top five principal components of CSF signal [34]. Images were smoothed with a 6 mm full-width at half-maximum kernel. More details on preprocessing are provided in Supplementary Methods.
Feature extraction
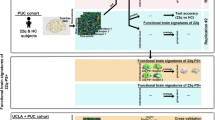
Time series from 434 regions-of-interest (ROIs) were extracted using a combination of functional and structural atlases: 400 ROIs matched to 17 large-scale resting-state networks from the Schaefer atlas (Fig. 1; [35]), 17 subcortical ROIs from the Harvard-Oxford Atlas [36] and 17 cerebellar ROIs from the Buckner 17-network atlas [37]. Time series from ROIs of participants with less than 80% voxel coverage were excluded, reducing the number of ROIs available for analysis (see Supplementary Methods). This procedure resulted in the exclusion of the amygdala and accumbens (ventral striatum) that are of particular interest for OCD. To incorporate these ROIs in the analysis, time series for these ROIs were extracted using 6-mm spheres around peak coordinates in NeuroSynth (neurosynth.org; [38]). A total of 2052 participants with time series from 318 ROIs with sufficient EPI coverage remained, which were used to extract different rs-fMRI features, including pairwise ROI-to-ROI functional connectivity (FC) and measures of local brain activity for each ROI: regional homogeneity (ReHo) measuring the temporal similarity of voxels with their neighbors [39], and fractional amplitude of low frequency fluctuations (fALFF), reflecting the intensity of spontaneous local brain activity [40]. In addition to these ROI-level features, we also calculated network-level FC (between-networks FC after averaging time series of ROIs in each network, and within-network FC for networks that included more than one ROI), and network-level ReHo and fALLF by taking the mean across ROIs from each network (see Supplementary Methods and Supplementary Table S3 for the included regions and network labels).
Parcels depict the 400 regions-of-interest (ROI) matched to 17 large scale resting-state networks from Schaefer and colleagues [35].
Mega-analyses
Linear Mixed Effect (LME) models were used to assess between-group differences, accounting for data clustering within samples with sample-varying effects, with diagnosis (fixed factor, OCD versus HC) as the variable of interest, age, sex, and head motion as covariates, and sample ID as a random intercept. Effect sizes (Cohen’s d) and significance (p values) were calculated for each ROI and network-level feature [41] (see Supplementary Methods). In our main analysis, we compared all OCD patients versus HC and compared adult and pediatric samples separately. Additionally, we performed stratified group analyses by comparing HC versus patients with and without current use of psychotropic medication at time of scanning, patients with lower severity (Y-BOCS < = 25; mild-moderate [42]) or higher severity of symptoms (Y-BOCS > 25; moderate-severe) based on the median, and adult patients with early (<18 years) and late (≥18 years) age of onset (AO), in line with prior ENIGMA-OCD mega-analyses [3, 25]. Samples with <10 participants per group were excluded for each analysis. Multiple comparisons correction (MCP) was applied for each modality separately (i.e., FC, ReHo and fALFF) using the two-stage Benjamini-Hochberg false discovery rate (FDR) procedure. qFDR for each modality was Bonferroni corrected (0.05/24 (12 contrasts x (ROI + network-wise features)) = 0.0020833) to account for the number of features and contrasts tested simultaneously.
Machine learning classification
Multivariate classifications were performed using linear Support Vector Machine (SVM) models implemented in scikit-learn (v1.0.2, in Python v3.9.5). Performance was evaluated using 20 times repeated stratified fivefold cross-validation (CV) and measured as the average area-under-the-receiver-operating-characteristic-curve (AUC). Stratified K-fold splits were made by preserving the proportion of patients and controls from each sample. Performance metrics were calculated for each CV iteration on the testing set and averaged across CV iterations. Hyper-parameters were optimized via nested grid-search across different values of C (0.001, 0.01, 0.1, 1, 10) using stratified fivefold CV on training data. Classifications were performed separately for all measures and group comparisons, consistent with previous mega-analyses. Statistical significance of classification performance was assessed using permutation testing with 1000 iterations [43], with Bonferroni correction for the number of classification contrasts for each measure separately (p < 0.05/24). Finally, we explored the influence of ComBat harmonization for removing site-effects in our best performing classifier (see Supplementary) [44].
Results
There was a significant difference in age (mean(SD) = 29.55(10.70) years for OCD; 27.98(9.97) for HC; t = 3.42, p < 0.001) and biological sex (%male=46.8 for OCD; 52.6 for HC; X2 = 7.02, p = 0.008), but corresponding effect sizes were small (d = 0.151 for age, phi = 0.058 for sex). Mean framewise displacement (FD) was significantly higher (t = 3.73, p < 0.001) in patients (mean(SD) = 0.11(0.05)) compared to HC (mean(SD) = 0.10(0.05)), but the proportion of high motion volumes (FD > 0.5 mm) was comparable (t = 1.70, p = 0.090). Data for subgroup analyses are reported in Supplementary Table S4. We included age, sex and mean FD as covariates in our LME models, and performed an additional sensitivity analysis with matched groups (described below). 48.5% of OCD patients used medication, 50.6% had a childhood-onset (<18 years), and the mean severity as assessed with the Y-BOCS was 24.92(6.41).
Main analysis
Compared to HC (N = 1028), OCD patients (N = 1024) showed widespread ROI-to-ROI hypo-connectivity (qFDR<0.05/24), with effect sizes ranging between −0.27 to −0.13 (Fig. 2; for individual ROI labels see Supplementary Fig. 2). ROI-to-ROI FC hypo-connections were predominantly located in sensorimotor (sub)networks, including bilateral primary sensorimotor cortex, supplementary motor area and central sulcus, in default mode (sub)networks (DMN) between bilateral precuneus/posterior cingulate cortex and dorsomedial prefrontal cortex (dmPFC), and between left ventrolateral (vl)PFC (including lateral orbitofrontal cortex) and bilateral dorsal (d)PFC, and in frontoparietal control (sub)networks (FPN; labeled as the “Control” network in the functional atlas) between regions surrounding the posterior cingulate gyrus. ROI-to-ROI hypo-connections between networks were found between bilateral precuneus (in DMN) and posterior cingulate gyrus (in FPN), left insula with right ventral (v)PFC, sensorimotor and salient/ventral attention (SN/VAN) networks, bilateral hippocampus with sensorimotor, and dorsal attention (DAN) and temporoparietal networks. We also found lower FC within bilateral thalamus and right thalamic hypo-connectivity with right caudate nucleus and posterior cingulate gyrus. The only significant hyper-connections were found between bilateral thalamus and right primary sensorimotor cortex and bilateral central sulcus, and between right medial (m)PFC and right extrastriate visual cortex (0.19 < d < 0.22). Notably, no significant differences in FC between the frontal cortex and striatum were observed. For network-wise FC, only the sensorimotor networks showed significant within-network hypo-connectivity, with an effect size of −0.18 (Supplementary Fig. 3).
Provided labels in this figure are for assigned networks only (individual ROI labels and ROI to network mapping can be found in Supplement). TempPar Temporal Parietal, Cont Frontoparietal Control, SalVentAttn Salience/Ventral Attention, DorsAttn Dorsal Attention, SomMot Sensorimotor, VisCent Visual Central (Visual A), VisPeri Visual Peripheral (Visual B).
For measures of local activity, patients showed lower ReHo in the right inferior extrastriate and left calcarine sulcus of the peripheral visual cortex (Visual B), and in the right parietal occipital cortex (−0.2 < d < −0.16) (Fig. 3A). For network-wise ReHo, patients showed lower ReHo in the peripheral visual cortex with an effect size of −0.14 (Supplementary Figure 4). Patients showed lower fALFF in the right calcarine sulcus and superior extrastriate of the peripheral visual cortex, bilateral sensorimotor cortex, right parieto-occipital cortex and bilateral postcentral gyri (−0.21 < d < −0.15) (Fig. 3B). For network-wise fALFF, patients showed lower fALFF in sensorimotor and DAN networks with effect sizes of −0.21 and −0.16 (Supplementary Figure 5).
Age
The pediatric sample consisted of 103 patients and 101 HC, and the adult sample of 903 patients and 913 HC. No significant differences were found between pediatric patients and HC for any of the measures. Adult patients showed widespread ROI-to-ROI hypo-connectivity, which broadly resembled that seen in the main analysis (−0.28 < d < −0.15), and additionally showed lower FC between left caudate and left posterior cingulate gyrus. Adult patients also showed hyper-connectivity between right thalamus and right central sulcus (consistent with the main analysis; 0.21 < d < 0.22). Additional hyperconnectivity was found between right lPFC and left post central gyrus, but no hyperconnectivity between medial PFC and extrastriate visual cortex was observed. The network-wise FC analysis showed additional hypo-connections in adult patients within and between sensorimotor networks, and lower connectivity between temporoparietal and visual central (Visual A) and between subcortical regions and cerebellar networks (−0.19 < d < −0.17). Comparisons in local connectivity between adult patients and HC were nearly identical to the findings of the main analysis, with lower ReHo (−0.21 < d < −0.18) in most of the same regions (though not in the right inferior extrastriate cortex). No significant differences were found for network-wise ReHo. Adult patients also showed similar but fewer regions with lower regional fALFF (−0.2 < d < −0.17), and identical networks with reduced fALFF (−0.2 < d < −0.16) (Supplementary Figures 6–11).
Medication
The comparison of 342 medicated patients to 509 HC showed ROI-to-ROI hypo-connectivity within and between sensorimotor networks and within and between temporoparietal networks, lower connectivity between left postcentral gyrus and right sensorimotor areas, right medial frontal cortex and right frontal operculum, left vlPFC (including lateral orbitofrontal cortex) and right mPFC and between right thalamus and right posterior cingulate gyrus. The number of significantly different connections was considerably smaller, but the effect sizes were approximately twice as large as for the entire sample, ranging from −0.38 to −0.3 (Fig. 4). Medicated patients also showed lower fALFF in the left central sulcus with an effect size of −0.33 (Supplementary Figure 12). No significant differences were found between medicated patients and HC for network-wise FC or fALFF, or regional and network-wise ReHo. The comparison between 356 unmedicated patients and 420 HC showed no significant group differences for any of the measures. However, the effect sizes for ROI-to-ROI hypo-connections that were found to be significant in the main analysis showed comparable effect sizes in unmedicated patients, ranging between −0.33 to −0.09. No significant differences between medicated and unmedicated patients were observed for any of the measures.
Provided labels in this figure are for assigned networks only (individual ROI labels and ROI to network mapping can be found in Supplement). TempPar Temporal Parietal, Cont Frontoparietal Control, SalVentAttn Salience/Ventral Attention, DorsAttn Dorsal Attention, SomMot Sensorimotor, VisCent Visual Central (Visual A), VisPeri Visual Peripheral (Visual B).
Symptom severity
The comparison of 376 low-severity patients (YBOCS < = 25) with 598 HC showed no significant group differences. The comparison of 281 high-severity patients with 470 HC showed ROI-to-ROI hypo-connectivity between left and right insula, between left insula and sensorimotor networks and within- and between sensorimotor networks (−0.37 < d < −0.3) (Fig. 5, Supplementary Fig. 13), and lower network-wise FC within sensorimotor networks (d = −0.34; Supplementary Fig. 14). High-severity patients also showed lower fALFF in sensorimotor networks for both regional and network-averaged features with effect sizes of −0.34 and −0.27, respectively (Supplementary Figs. 15, 16). No significant differences were detected for regional or network-wise ReHo, and no significant differences between low and high severity patients were observed for any of the measures.
Provided labels in this figure are for assigned networks only (individual ROI labels can be found in Supplement). TempPar Temporal Parietal, Cont Frontoparietal Control, SalVentAttn Salience/Ventral Attention, DorsAttn Dorsal Attention, SomMot Sensorimotor, VisCent Visual Central (Visual A), VisPeri Visual Peripheral (Visual B).
Age of onset
For early-onset (age<18) patient sub-analyses, we included 198 adult patients and 383 HC, and group comparisons showed no significant differences. For late AO patient sub-analyses, we included 300 adult patients and 473 HC. Late AO patients showed lower ROI-to-ROI connectivity between left and right sensorimotor cortical areas (−0.34 < d < −0.33) and lower network-wise FC within somatomotor and DMN with effect sizes of −0.25 and −0.34 (Supplementary Figs. 17–18). No significant differences were found for fALFF and ReHo features, and no significant differences between early and late onset patients were observed for any of the measures.
Sensitivity analysis
To assess whether significant differences in age, sex, and mean FD influenced the results, we repeated our main analysis for ROI-to-ROI FC in an age, sex and mean FD matched sample using propensity score matching (see Supplementary). 811 patients and 797 HC were included (N = 1608 subjects from 25 samples). Patients showed widespread ROI-to-ROI hypo-connectivity which largely resembled those seen in main analysis (−0.31 < d < −0.15), however the number of significant hypo-connections increased by 64% (N = 319 for matched sample comparison, N = 194 for main analyses) (Supplementary Figs. 19-20). Notably, 89% of hypo-connections detected in the main analyses remained significant, but three (out of four) hippocampal hypo-connections with sensorimotor, and dorsal attention (DAN) and temporoparietal networks did not. Instead, new significant hypo-connections were found between bilateral hippocampi and regions within sensorimotor networks, as well as cortical—basal ganglia hypo-connections between bilateral caudate and left posterior cingulate, and between left pallidum and right temporal parietal cortex. The hyper-connectivity between regions detected in our main analysis were no longer significant in the matched sample after MCP correction at qFDR = 0.05/24, but these hyper-connections did attain significance at qFDR = 0.05 (0.001<pcorrected < 0.02) with comparable effect sizes (0.17 < d < 0.21).
Machine learning
First, we assessed SVM classification performance to distinguish OCD patients from HC using the complete sample. Overall performance was low though significant, with AUC (averaged across CV folds and repeats) ranging between 0.567 and 0.673 across the different measures (Fig. 6). The best performance was achieved using ROI-to-ROI FC (pcorrected = 0.024). Classification for adult patients versus HC led to similar performance, with AUCs ranging between 0.565 and 0.684. Performance for pediatric patients versus HC was poor (AUC: 0.542–0.615) and did not reach significance. Classification performance for medicated OCD versus HC using ROI-to-ROI-FC led to an AUC of 0.702 (pcorrected = 0.024), whereas the classification between unmedicated OCD versus HC was 0.608 (pcorrected = 0.024). All other classifications (i.e., performed separately for low and high severity, and early and late AO patients versus HC) performed lower than 0.70 AUC. To summarize, twenty seven out of the additional 34 classifications of OCD subgroups versus controls were significant, and seven out of 18 between patient group classifications were significant. A complete overview of classification results, including balanced accuracy, sensitivity, specificity and significance assessed with labels permutations are provided in Supplementary Table S5. We further assessed whether ComBat harmonization improved the performance of our best performing classifier on the complete sample (i.e., using ROI-to-ROI FC). Post-ComBat, the SVM was no longer able to classify the sample of origin above chance level, indicating the successful removal of site-effects. This came at a slight cost in the classification performance for diagnosis and sex (see Supplementary).
Discussion
Our mega-analyses demonstrated widespread FC aberrations in OCD patients, with global hypo-connectivity and only few hyper-connections. Notably, most of the significant hypo-connections were located within the sensorimotor network. Brain regions involved in the altered connectivity patterns partly correspond to current pathophysiological models of OCD which are mainly based on other neuroimaging modalities [5, 8, 11]. However, our results indicate a lesser degree of subcortical involvement in OCD as measured by resting-state functional MRI, and we did not observe differences in fronto-striatal FC, and only few differences in basal ganglia FC that are central to those models (i.e. between the thalamus and caudate). These results suggest that neural models of OCD should be revised to incorporate hypo-connectivity of the sensorimotor network in particular. Furthermore, despite global hypo-connectivity at the group level, our machine learning results showed that FC could not provide an accurate distinction between OCD patients and HC. Overall classification performance was poor, with AUCs ranging between 0.567–0.673 across the different features when trained on the complete sample, which is insufficient for clinical application [45, 46].
This is the largest FC analysis in OCD conducted to date. Our most consistent finding was lower connectivity within cortical sensorimotor (sub)networks, detected for the majority of OCD versus HC comparisons for both ROI and network-wise FC. The sensorimotor network is often overlooked in OCD studies. There has been some prior evidence OCD related FC alterations in this network [6,7,8, 14, 16, 47,48,49]. However, recent meta-analyses on seed-based FC studies in OCD have not reported conclusive findings for the sensorimotor cortex, as studies rarely include seeds within these regions for analysis [20, 22]. The sensorimotor cortex is involved in the generation and control of motor behaviors and integration of sensory information [8]. Alterations in this network could be related to sensory phenomena, aversive or uncomfortable tactile sensations, or perceptions that drive repetitive behaviors [7, 50, 51]. The network is integrated with the sensorimotor CSTC circuit that is relevant to OCD because of its important role in habit formation [6,7,8]. Altered connectivity within the sensorimotor areas could also reflect impaired sensorimotor gating, the process of suppressing irrelevant sensory, cognitive and motor information to facilitate mental and behavioral integration and flexibility [12]. This could contribute to the inability to inhibit undesired thoughts and images and repetitive behaviors or mental acts [13,14,15]. The sensorimotor circuit also participates in functions of other neural circuits and vice versa, with the insula and fronto-limbic structures engaged during emotional processing, and early habit formation relying on reward signaling in the ventral affective circuit [7]. Interestingly, a recent transdiagnostic study identified dysconnectivity within the sensorimotor network related to general psychopathology and impulsivity, suggesting that sensorimotor processes affect symptomatology and cognitive function across multiple disorders [52].
In line with previous literature, we identified hypo-connectivity within and between networks of the triple network model i.e., DMN, FPN, SN [20, 21]. The impaired interplay between DMN, FPN, SN could translate into difficulties switching between unwanted, repetitive thoughts and/or compulsions and meaningful, goal-directed action. Secondly, we found aberrant connectivity within CSTC circuits, including thalamic hypo-connectivity with the ventral striatum (including caudate nucleus) and posterior cingulate gyrus regions, and prefrontal hypo-connectivity between CSTC circuits. Our sensitivity analysis using an age, sex and motion matched sample did detect additional cortical—basal ganglia hypo-connections between bilateral caudate and left posterior cingulate, and between left pallidum and right temporal parietal cortex. However, we did not find differences in FC connectivity between prefrontal cortical regions and striatal regions like the putamen, pallidum, caudate nucleus and accumbens, nor did we find FC differences for the amygdala. Of the few FC hyper-connections we observed, most involved connections between (bilateral) thalamus and primary sensorimotor cortex and central sulcus embedded in the sensorimotor CSTC circuit. Recent views on the thalamus hold that it is not a passive relay station but that it has a central role in modulating cortical functioning [53]. This could suggest that thalamic hyperconnectivity with sensorimotor cortical areas may disrupt higher-order corticocortical connectivity within sensorimotor (sub)networks. Interestingly, a recent ENIGMA-OCD study also demonstrated thalamic aberrations, with unmedicated pediatric patients showing larger volumes and adults showing smaller volumes compared to controls [54]. These findings provide further support that the thalamus is a crucial hub in the CSTC circuits and plays a vital role in the pathophysiology underlying OCD. Apart from altered sensorimotor CSTC connectivity, we also found lower connectivity in regions connected to the fronto-limbic CSTC circuit, namely between insula and vPFC, and between cingulate areas and hippocampus, which may be related to altered emotion regulation in OCD [7, 8].
In this study, we also investigated additional rs-fMRI features that describe the intensity of spontaneous brain activity (fALFF) and local synchronization (ReHo). These measures are calculated in a voxel-wise manner and are considered to be reflective of local brain properties, whereas long-range distance correlations (i.e. ROI-to-ROI FC) measure the functional connections between distant brain regions. These measures provide complementary information about the functional organization of the brain and allow for a more detailed and nuanced understanding of brain function. Group differences in measures of local brain activity and synchronization were partly consistent with FC results. OCD patients showed lower fALFF in sensorimotor, visual periphery and dorsal attention network areas, whereas for ReHo patients showed lower activity in dorsal attention network and visual periphery, but not in sensorimotor areas. This is in line with studies reporting lower fALFF and reHo in the sensorimotor cortex and occipital areas [55,56,57,58]. However, we did not detect any regions with higher ReHo or fALFF, which have been reported in previous studies [55, 56, 59, 60]. These inconsistencies suggest that the results from these smaller studies might not generalize well to larger samples.
In our secondary analyses we investigated differences between subgroups of participants with specific demographical and clinical characteristics (i.e. age groups, severity of symptoms, age of onset and medication status). No significant case-control differences were detected except for comparisons between adult samples (with changes in FC similar to those seen in our main analyses), and those between late-onset, high-severity and medicated patients versus HC. Few larger scale resting-state studies have investigated the effects of the aforementioned characteristics in OCD. A recent meta-analysis of 47 studies used meta-regression and found a significant negative correlation between the mean age of patients and caudate hypo-connectivity [22]. This is in line with our findings, adult patients showed lower FC between left caudate and posterior cingulate gyrus and this not detected in our main comparisons that included pediatric patients. The meta-regression also showed a negative correlation between age of onset and thalamic-putamen hypo-connectivity, and a negative correlation between symptom severity and hypo-connectivity between the nucleus accumbens and medial orbitofrontal cortex [22]. We did not detect hypo-connectivity between these regions in our age on onset and severity subgroups analyses. Group differences for late-onset adult patients compared to controls were sparse and limited to lower ROI-to-ROI FC in sensorimotor networks, and high severity patients showed only a few hypo-connections, located in sensorimotor networks and within insula regions. This discrepancy in findings could relate to methodological differences between studies. For example, we investigated stratified subgroups rather than regression in line with previous ENIGMA-OCD mega-analyses. Additionally, we used a mega-analytic approach by pooling individual data across studies rather than a meta-analytic approach that synthesizes summary statistics to estimate an overall effect. Our null findings for other case-control comparisons (i.e. pediatric samples, early onset and low severity patients) indicate that these subgroups have no clear association with functional connectivity, but this might also be related to a lack of statistical power as these analyses included fewer patients (N = 103, 198 and 376, respectively).
We found the largest effect sizes for differences between medicated patients versus HC, and these were mainly located in sensorimotor regions. We did not detect any significant differences between unmedicated patients and controls. However, exploratory analyses showed that effect sizes for the comparison between unmedicated patients versus controls were comparable with the main analysis. This suggests that findings from the main analysis are not fully driven by medication effects. Furthermore, the lack of significance after multiple comparisons correction might be explained by reduced power due to a smaller sample size compared to our main analysis, and smaller associated effect sizes in unmedicated patients versus controls compared to medicated patients versus controls. These results with larger differences for medicated than unmedicated patients were corroborated by the machine learning analyses, which showed higher classification performance for medicated (0.702 AUC) than unmedicated patients versus control (0.608 AUC). This difference in performance might be explained by medication specific effects that could alter brain structure and function, which would possibly allow for better discrimination between groups. Findings from rodent studies indicate that serotonin reuptake inhibitors (SRIs) promote neuroplasticity in the cortex and subcortex through glio-genesis and neurogenesis [61,62,63]. However, it is unclear whether these findings translate to humans, and the impact of long-term medication use is not well understood [64]. The influence of medication on FC resembles previous work from ENIGMA-OCD on brain structure; thinner cortices (in adults) and smaller surface areas (in children) were restricted to medicated patients [3, 25]. Similarly, smaller thalamic volume in adults [54] and microstructural alterations in white matter in OCD were mainly driven by medicated patients. Previous ENIGMA-OCD classification analyses based on structural MRI pointed in the same direction, with low overall classification performance between all patients versus controls (AUC = 0.57–0.62) and higher classification performance for medicated patients than for unmedicated patients versus controls (AUC = 0.69 and 0.60, respectively) [26]. But while classification between medicated and unmedicated patients based on structural MRI reached over 0.80 AUC [26], the same classification in this study was 0.56–0.64 AUC. In addition, univariate analyses in the current study showed no significant differences between medication groups. These findings suggest that although the functional differences in OCD are more pronounced in medicated patients, they are not as specific as for brain structure. Little is known about the effects of psychotropic medication on resting-state connectivity in OCD. While meta-analysis of cerebral blood flow and metabolism studies suggests that treatment with SRIs decreases resting caudate nucleus and orbitofrontal cortex activity [65], small-scale FC studies have reported increased striatal connectivity and graph measures of whole-brain connectivity after treatment [66, 67]. However, placebo-controlled studies in healthy volunteers suggest that SRIs primarily reduce FC [68, 69], suggesting that FC normalization after treatment may reflect symptom improvement rather than direct SRI effects. Additional longitudinal studies are warranted to better understand the effects of medication on FC in OCD.
The obtained classification performances in this study are in line with previous work by the ENIGMA-OCD working group using structural MRI. We suspect that the poor overall classification performance is related to the neurobiological heterogeneity underpinning OCD, also related to developmental stage and disease stage [6]. Also, patients can present a variety of symptoms in different combinations, each of which may be caused by distinct brain changes [7]. It is possible that there is no universal biomarker that would be effective for all patients [24]. This heterogeneity is likely to be further exacerbated in a large multicenter study like ENIGMA that combines samples with different scanning parameters, processing pipelines, inclusion criteria and demographic- and other clinical characteristics [70]. In contrast, prior studies using MRI to classify OCD with smaller monocenter samples have reached accuracies up to 93% [18]. These smaller samples are often more homogeneous and use carefully selected patients based on specific inclusion and exclusion criteria to enhance statistical power, but they may not be representative of the broader clinical population. Thus, whereas small monocenter studies focus on answering a specific question about their patient population, large multicenter studies assume that a fundamental pattern of the disorder of interest can be detected despite the presence of heterogeneity, and both are geared toward answering complementary questions [23, 24, 26, 71, 72]. Future classification studies using a similar multicenter setting to our own could investigate the feasibility of using multimodal data (i.e. a combining functional, structural and/or diffusion tensor imaging MRI), deep learning techniques and the use of unsupervised techniques to address phenotypic heterogeneity seen in clinical populations and to stratify disorders into more meaningful subgroups.
Several limitations should be noted. First, we used a retrospectively pooled sample from existing studies across the world, with different scanners and no harmonized data collection protocols. fMRI data were collected with different scanning durations and temporal and spatial resolutions which may affect the signal-to-noise of images and contribute to the heterogeneity of the data. However, we addressed differences between centers through the use of random intercepts in our LME models and Combat harmonization for the machine learning analysis. And whereas variability in fMRI data collection can be viewed as a limitation, it can also be considered a strength as we were able to test if results are robust across diverse protocols and hardware. Second, age, sex, and head motion were significantly different between groups. However, these differences were small in terms of effect size and all performed LME models included these variables as additional covariates in our analyses. In addition, a sensitivity analysis with matched samples showed even more FC differences between groups, suggesting that the results were not driven by group differences in age, sex and head motion. Third, the cross-sectional design of this study did not allow for reliable investigation of medication effects, and limited information was available on history, type, dosage and duration of treatment. Longitudinal studies that incorporate more detailed medication information may provide better insight into the long and short-term effects of medication on FC. Finally, there is a lack of information on OCD subtypes in our dataset. Particular OCD subtypes may be characterized by different neural correlates, and this might limit the ability of machine learning models to find generalizable patterns in brain structure and function [18, 26, 73]. Further studies including detailed clinical information are needed to disentangle this issue.
Taken together, our findings provide evidence for large-scale network aberrations in OCD and highlight the importance of sensorimotor network hypo-connectivity, which should be incorporated into neural models of OCD. Despite abundant hypoconnectivity, univariate effect sizes were small and multivariate classification performance was poor. This indicates that FC does not currently provide an accurate biomarker for OCD, presumably due to disease heterogeneity.
Code availability
The computer code for the above-described analyses will be provided upon reasonable request.
Change history
15 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41380-023-02211-y
References
Torres AR, Prince MJ, Bebbington PE, Bhugra D, Brugha TS, Farrell M, et al. Obsessive-compulsive disorder: prevalence, comorbidity, impact, and help-seeking in the British National Psychiatric Morbidity Survey of 2000. Am J Psychiatry. 2006;163:1978–85.
Association AP. Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric A, American Psychiatric Association DSMTF, editors. Arlington, VA: American Psychiatric Association; 2013.
Boedhoe PS, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, et al. Distinct subcortical volume alterations in pediatric and adult OCD: a worldwide meta- and mega-analysis. Am J Psychiatry. 2017;174:60–9.
Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–49.
Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43–51.
Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, et al. Obsessive–compulsive disorder. Nat Rev Dis Prim. 2019;5:52.
Shephard E, Stern ER, van den Heuvel OA, Costa DLC, Batistuzzo MC, Godoy PBG, et al. Toward a neurocircuit-based taxonomy to guide treatment of obsessive-compulsive disorder. Mol Psychiatry. 2021;26:4583–604.
van den Heuvel OA, van Wingen G, Soriano-Mas C, Alonso P, Chamberlain SR, Nakamae T, et al. Brain circuitry of compulsivity. Eur Neuropsychopharmacol. 2016;26:810–27.
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81.
Pauls DL, Abramovitch A, Rauch SL, Geller DA. Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat Rev Neurosci. 2014;15:410–24.
Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron 2000;28:343–7.
Cromwell HC, Mears RP, Wan L, Boutros NN. Sensory gating: a translational effort from basic to clinical science. Clin EEG Neurosci. 2008;39:69–72.
Hoexter MQ, Biazoli CE Jr., Alvarenga PG, Batistuzzo MC, Salum GA, Gadelha A, et al. Low frequency fluctuation of brain spontaneous activity and obsessive-compulsive symptoms in a large school-age sample. J Psychiatr Res. 2018;96:224–30.
Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology 2012;37:1216–23.
Moreira PS, Marques P, Magalhaes R, Esteves M, Sousa N, Soares JM, et al. The resting-brain of obsessive-compulsive disorder. Psychiatry Res Neuroimaging. 2019;290:38–41.
Sha Z, Edmiston EK, Versace A, Fournier JC, Graur S, Greenberg T, et al. Functional disruption of cerebello-thalamo-cortical networks in obsessive-compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:438–47.
Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med. 1995;34:537–41.
Bruin W, Denys D, van Wingen G. Diagnostic neuroimaging markers of obsessive-compulsive disorder: Initial evidence from structural and functional MRI studies. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:49–59.
Parkes L, Satterthwaite TD, Bassett DS. Towards precise resting-state fMRI biomarkers in psychiatry: synthesizing developments in transdiagnostic research, dimensional models of psychopathology, and normative neurodevelopment. Curr Opin Neurobiol. 2020;65:120–8.
Gursel DA, Avram M, Sorg C, Brandl F, Koch K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev. 2018;87:151–60.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506.
Liu J, Cao L, Li H, Gao Y, Bu X, Liang K, et al. Abnormal resting-state functional connectivity in patients with obsessive-compulsive disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;135:104574.
Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Reproducible brain-wide association studies require thousands of individuals. Nature 2022;603:654–60.
Gratton C, Nelson SM, Gordon EM. Brain-behavior correlations: two paths toward reliability. Neuron 2022;110:1446–9.
Boedhoe PSW, Schmaal L, Abe Y, Alonso P, Ameis SH, Anticevic A, et al. Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: findings from the ENIGMA Obsessive-Compulsive Disorder Working Group. Am J Psychiatry. 2018;175:453–62.
Bruin WB, Taylor L, Thomas RM, Shock JP, Zhutovsky P, Abe Y, et al. Structural neuroimaging biomarkers for obsessive-compulsive disorder in the ENIGMA-OCD consortium: medication matters. Transl Psychiatry. 2020;10:342.
Orru G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using support vector machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci Biobehav Rev. 2012;36:1140–52.
Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown obsessive compulsive scale: I. Development, use, and reliability. Arch Gen psychiatry. 1989;46:1006–11.
Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, et al. Children’s Yale-Brown obsessive compulsive scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–52.
Waller L, Erk S, Pozzi E, Toenders YJ, Haswell CC, Buttner M, et al. ENIGMA HALFpipe: Interactive, reproducible, and efficient analysis for resting-state and task-based fMRI data. Hum Brain Mapp. 2022;43:2727–42.
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–6.
Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–77.
Pruim RHR, Mennes M, Buitelaar JK, Beckmann CF. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage. 2015;112:278–87.
Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage 2014;96:22–35.
Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018;28:3095–114.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80.
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–45.
Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70.
Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400.
Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–41.
Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605.
Storch EA, De Nadai AS, Conceição do Rosário M, Shavitt RG, Torres AR, Ferrão YA, et al. Defining clinical severity in adults with obsessive–compulsive disorder. Compr Psychiatry. 2015;63:30–5.
Ojala M, Garriga GC. Permutation tests for studying classifier performance. J Mach Learn Res. 2010;11:6.
Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 2018;167:104–20.
Hosmer Jr DW, Lemeshow S, Sturdivant RX. Applied logistic regression: John Wiley & Sons; 2013.
First M, Botteron K, Carter C, Castellanos FX, Dickstein DP, Drevets W, et al. Consensus Report of the APA Work Group on Neuroimaging markers of Psychiatric Disorders. Am Psychiatr Assoc. 2012. Available at https://www.psychiatry.org/File%20Library/Psychiatrists/Directories/Library-and-Archive/resource_documents/rd2012_Neuroimaging.pdf.
Stern ER, Eng GK, De Nadai AS, Iosifescu DV, Tobe RH, Collins KA. Imbalance between default mode and sensorimotor connectivity is associated with perseverative thinking in obsessive-compulsive disorder. Transl Psychiatry. 2022;12:19.
Cui G, Ou Y, Chen Y, Lv D, Jia C, Zhong Z, et al. Altered global brain functional connectivity in drug-naive patients with obsessive-compulsive disorder. Front Psychiatry. 2020;11:98.
Li X, Li H, Jiang X, Li J, Cao L, Liu J, et al. Characterizing multiscale modular structures in medication-free obsessive-compulsive disorder patients with no comorbidity. Hum Brain Mapp. 2022;43:2391–9.
Subira M, Sato JR, Alonso P, do Rosario MC, Segalas C, Batistuzzo MC, et al. Brain structural correlates of sensory phenomena in patients with obsessive-compulsive disorder. J Psychiatry Neurosci. 2015;40:232–40.
Brown C, Shahab R, Collins K, Fleysher L, Goodman WK, Burdick KE, et al. Functional neural mechanisms of sensory phenomena in obsessive-compulsive disorder. J Psychiatr Res. 2019;109:68–75.
Kebets V, Holmes AJ, Orban C, Tang S, Li J, Sun N, et al. Somatosensory-motor dysconnectivity spans multiple transdiagnostic dimensions of psychopathology. Biol Psychiatry. 2019;86:779–91.
Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci. 2016;19:533–41.
Weeland CJ, Kasprzak S, de Joode NT, Abe Y, Alonso P, Ameis SH, et al. The thalamus and its subnuclei-a gateway to obsessive-compulsive disorder. Transl Psychiatry. 2022;12:70.
Xia J, Fan J, Liu W, Du H, Zhu J, Yi J, et al. Functional connectivity within the salience network differentiates autogenous- from reactive-type obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2020;98:109813.
Gimenez M, Guinea-Izquierdo A, Villalta-Gil V, Martinez-Zalacain I, Segalas C, Subira M, et al. Brain alterations in low-frequency fluctuations across multiple bands in obsessive compulsive disorder. Brain Imaging Behav. 2017;11:1690–706.
Ping L, Su-Fang L, Hai-Ying H, Zhang-Ye D, Jia L, Zhi-Hua G, et al. Abnormal spontaneous neural activity in obsessive-compulsive disorder: a resting-state functional magnetic resonance imaging study. PLoS One. 2013;8:e67262.
Hao H, Chen C, Mao W, Xia W, Yi Z, Zhao P, et al. Alterations in resting-state local functional connectivity in obsessive-compulsive disorder. J Affect Disord. 2019;245:113–9.
Qing X, Gu L, Li D. Abnormalities of localized connectivity in obsessive-compulsive disorder: a voxel-wise meta-analysis. Front Hum Neurosci. 2021;15:739175.
Hu X, Zhang L, Bu X, Li H, Li B, Tang W, et al. Localized connectivity in obsessive-compulsive disorder: an investigation combining univariate and multivariate pattern analyses. Front Behav Neurosci. 2019;13:122.
Kodama M, Fujioka T, Duman RS. Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry. 2004;56:570–80.
Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–503.
Soumier A, Banasr M, Lortet S, Masmejean F, Bernard N, Kerkerian-Le-Goff L, et al. Mechanisms contributing to the phase-dependent regulation of neurogenesis by the novel antidepressant, agomelatine, in the adult rat hippocampus. Neuropsychopharmacology. 2009;34:2390–403.
Homberg JR, Schubert D, Gaspar P. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharm Sci. 2010;31:60–5.
van der Straten AL, Denys D, van Wingen GA. Impact of treatment on resting cerebral blood flow and metabolism in obsessive compulsive disorder: a meta-analysis. Sci Rep. 2017;7:17464.
Shin DJ, Jung WH, He Y, Wang J, Shim G, Byun MS, et al. The effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:606–14.
Bernstein GA, Cullen KR, Harris EC, Conelea CA, Zagoloff AD, Carstedt PA, et al. Sertraline effects on striatal resting-state functional connectivity in youth with obsessive-compulsive disorder: a pilot study. J Am Acad Child Adolesc Psychiatry. 2019;58:486–95.
van Wingen GA, Tendolkar I, Urner M, van Marle HJ, Denys D, Verkes RJ, et al. Short-term antidepressant administration reduces default mode and task-positive network connectivity in healthy individuals during rest. Neuroimage. 2014;88:47–53.
McCabe C, Mishor Z. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. Neuroimage. 2011;57:1317–23.
Schnack HG, Kahn RS. Detecting neuroimaging biomarkers for psychiatric disorders: sample size matters. Front Psychiatry. 2016;7:50.
Nunes A, Schnack HG, Ching CRK, Agartz I, Akudjedu TN, Alda M, et al. Using structural MRI to identify bipolar disorders − 13 site machine learning study in 3020 individuals from the ENIGMA Bipolar Disorders Working Group. Mol Psychiatry. 2020;25:2130–43.
Thomas RM, Bruin W, Zhutovsky P, van Wingen G. Dealing with missing data, small sample sizes, and heterogeneity in machine learning studies of brain disorders. Machine learning: Elsevier; 2020. 249-66.
Mataix-Cols D, van den Heuvel OA. Common and distinct neural correlates of obsessive-compulsive and related disorders. Psychiatr Clin North Am. 2006;29:391–410. viii
Acknowledgements
The ENIGMA-Obsessive Compulsive Disorder Working-Group gratefully acknowledges support from the Amsterdam Neuroscience (CIA-2019-03-A to OAvdH, Amsterdam Neuroscience Alliance Project to GAvW), and the NIH Big Data to Knowledge (BD2K) award for foundational support and consortium development (U54 EB020403 to PMT). For a complete list of ENIGMA-related grant support please see here: http://enigma.ini.usc.edu/about-2/funding/. Additional funding was supported by the Japan Society for the Promotion of Science (JSPS; KAKENHI Grant No. 18K15523 to YA, Grant No. 22H01090, 21K03084, 19K03309, 16K04344 to YH, Grant No. (C)21K07547, 22K07598 and 22K15766 to TN and HT); the Carlos III Health Institute (Grant No. PI18/00856 to PA, Grant No. CM21/00278 (co-funded by the European Social Fund) to SBT, Grant No. FI17/00294 to IM-Z, Grant No. PI19/01171 to CS-M); the National Institute of Mental Health (NIMH; Grant No. 5R01MH116038 to AA, Grant No. R01MH085900 and R01MH121520 to JDF, Grant No. R01MH117601, R01AG059874, P41EB015922, R01MH126213 and R01MH121246 to NJ, Grant No. K23 MH115206 to PG, Grant No. R21MH101441 to RM, Grant No. R21MH093889 and R01MH104648 to RM and BHS, Grant No. R01MH085900 to JO’N, Grant. No. R01MH081864 to JO’N and JP, Grant No. K24MH121571 to CP, Grant No. R01MH126981, R01MH111794 and R33MH107589 to ERS, Grant No. R01MH116147, P41EB015922, and R01MH123163 to PMT); the Hartmann Müller Foundation (Grant No. 1460 to SB); the International Obsessive-Compulsive Disorder Foundation (IOCDF) Research Award to PG; the Japan Agency for Medical Research and Development (AMED Brain/MINDS Beyond program Grant No. JP22dm0307002 to YH and Grant No. JP22dm0307008 to YS); Michael Smith Foundation for Health Research to FJ-F; the Deutsche Forschungsgemeinschaft (DFG; Grant No. KO 3744/11-1 to KK, Grant NO. VE 892/2-1 to NCV and LLB); the Heidehof Stiftung to NCV; the Marató TV3 Foundation (Grant No. 091810 to LL); the Fondo de Investigaciones Sanitarias, of the Spanish Ministry of Health (Grant No. PI11/01419 to LL); the National Research Foundation of South Africa to CL; the South African Medical Research Council (SAMRC) to DJS and CL; the Portuguese Foundation for Science and Technology (Fundação para a Ciência e a Tecnologia; FCT; Grant No. 2020.07946.BD to NS, Grant No. UIDB/50026/2020 and UIDP/50026/2020 to PM); the Norte Portugal Regional Operational Programme (NORTE 2020; Grant No. NORTE-01-0145-FEDER-000013 and NORTE-01-0145-FEDER-000023 to PM and NS), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF); the FLAD Science Award Mental Health 2021 to PM and NS; the Government of India grants from the Department of Science and Technology (Grants No. SR/S0/HS/0016/2011 to YCJR, and DST INSPIRE faculty Grant No. IFA12-LSBM-26 to JCN) and from the Department of Biotechnology (Grant No. BT/ PR13334/Med/30/259/2009 to YCJR, and Grant No. BT/06/IYBA/2012 to JCN); the Spanish Ministry of Universities with funds from the European Union—NextGenerationEU (Grant No. MAZ/2021/11 to MP-P); the National Council for Scientific and Technological Development (CNPq, Grant No. 303754/2018-4 to RGS); the Italian Ministry of Health (IRCCS Fondazione Santa Lucia Ricerca Corrente 2022, 2023 to FP, Grant No. RC19-20-21-22/A to GS); the Wellcome Trust-DBT India Alliance Senior Fellowship (Grant No. 500236/Z/11/Z to GV), Early Career Fellowship (Grant No. IA/CPHE/18/1/503956 to VS) and Clinical and Public Health Research Center grants (Grant No. IA/CRC/19/1/610005 to GV); the Catalan Agency for the Management of University and Research Grants (AUGUR Grant No. 2017SGR 1247 to CS-M); the National Natural Science Foundation of China (Grant No. 81871057 and 82171495 to JT, Grant No. 82071518 to ZW), and Key Technologies Research and Development Program of China (Grant No. 2022YFE0103700 to JT); the Obsessive-Compulsive Foundation to DJS; the Helse Vest Health Authority (Grant No. 911754 and 911880 to ALT); the Else-Kröner-Fresenius Foundation (Grant No. 2017_A101 to NW); the Swiss National Science Foundation (Grant No. 320030_130237 to SW); Netherlands Organization for Scientific Research (NWO/ZonMW Vidi Grant No. 165.610.002, 016.156.318, and 917.15.318 to GAvW, Grant No. 916.86.038 to OAvdH); The Brain & Behavior Research Foundation (NARSAD grant) and Netherlands Brain Foundation (Grant No. 2010(1)−(50)) to OAvdH and the National Institute on Aging Research Project Grant Program (Grant No. R01AG058854 to YDvdW) and the ENIGMA Parkinson’s Initiative: A Global Initiative for Parkinson’s Disease, NINDS award RO1NS107513 to YDvdW.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: WBB, GAvW, OAvdH, DJS, PMT. Methodology: WBB, GAvW. Software: WBB, LW, IMV. Formal analysis: WBB. Investigation: WBB, YA, PA, AA, LLB, SB, NB, MCB, FB, SBT, SB, FC, BC, DAJPD, MANE, GKE, SF, JDF, RGG, PG, JYG, KH, BH, YH, MQH, NJ, FJ, SK, MK, KK, YBK, JSK, LL, CRL, CL, RM, IM, JMM, PSM, PM, AN, TN, JCN, ELN, JCPZ, JP, MP, FP, FP, CP, JYCR, DR, YS, ES, VS, BHS, CS, NS, GS, ERS, SES, PRS, JT, SIT, ALT, YT, HT, BV, IMV, GV, NCV, CV, SW, LW, ZW, AW, NW, JY, QZ, WAvL, HJFvM, LAvdM, AvdS, YDvdW, PMT, DJS, OAvdH, GAvW. Data Curation: WBB, YA, PA, AA, LLB, SB, NB, MCB, FB, SBT, SB, FC, BC, DAJPD, MANE, GKE, SF, JDF, RGG, PG, JYG, KH, BH, YH, MQH, NJ, FJ, SK, MK, KK, YBK, JSK, LL, CRL, CL, RM, IM, JMM, PSM, PM, AN, TN, JCN, ELN, JCPZ, JP, MP, FP, FP, CP, JYCR, DR, YS, ES, VS, BHS, CS, NS, GS, ERS, SES, PRS, JT, SIT, ALT, YT, HT, BV, IMV, GV, NCV, CV, SW, LW, ZW, AW, NW, JY, QZ, WAvL, HJFvM, LAvdM, AvdS, YDvdW, PMT, DJS, OAvdH, GAvW. Writing - Original Draft: WBB. Writing - Review & Editing: WBB, YA, PA, AA, LLB, SB, NB, MCB, FB, SBT, SB, FC, BC, DAJPD, MANE, GKE, SF, JDF, RGG, PG, JYG, KH, BH, YH, MQH, NJ, FJ, SK, MK, KK, YBK, JSK, LL, CRL, CL, RM, IM, JMM, PSM, PM, AN, TN, JCN, ELN, JCPZ, JP, MP, FP, FP, CP, JYCR, DR, YS, ES, VS, BHS, CS, NS, GS, ERS, SES, PRS, JT, SIT, ALT, YT, HT, BV, IMV, GV, NCV, CV, SW, LW, ZW, AW, NW, JY, QZ, WAvL, HJFvM, LAvdM, AvdS, YDvdW, PMT, DJS, OAvdH, GAvW. Visualization: WBB. Supervision: GAvW, OAvdH, DJS, PMT.
Corresponding authors
Ethics declarations
Competing interests
AA consults and holds equity with Neumora Therapeutics (formerly BlackThorn Therapeutics) and Manifest Technologies. AA also serves on the technology advisory board of Neumora Therapeutics and on the board of directors for Manifest Technologies. AA is a co-inventor on the following patents: AA, Murray JD, Ji JL: Systems and Methods for Neuro-Behavioral Relationships in Dimensional Geometric Embedding (N-BRIDGE), PCT International Application No. PCT/US2119/022110, filed March 13, 2019 and Murray JD, AA, Martin, WJ: Methods and tools for detecting, diagnosing, predicting, prognosticating, or treating a neurobehavioral phenotype in a subject, U.S. Application No.16/149, filed on October 2, 2018, U.S. Application for PCT International Application No.18/054, 009 filed on October 2, 2018. JDF received consultant fees from NOCD, Inc. PM has received in the past 3 years grants, CME-related honoraria, or consulting fees from Angelini, AstraZeneca, Bial Foundation, Biogen, DGS-Portugal, FCT, FLAD, Janssen-Cilag, Gulbenkian Foundation, Lundbeck, Springer Healthcare, Tecnimede and 2CA-Braga. ELN disclosed that she is an unpaid advisory board member of Tourette Association of America and Myriad Genetics. In the last three years, BHS has received royalties from UpToDate, Inc, and Cambridge University Press and a stipend from the American Medical Association for serving as Associate Editor of JAMA-Psychiatry. PMT received unrelated research grant support from Biogen, Inc. All other individually-named authors in- and outside of the ENIGMA-OCD working group reported no biomedical financial interests or potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article Honami Arai, Irene Bollettini, Rosa Calvo Escalona, Ana Coelho, Federica Colombo, Leila Darwich, Martine Fontaine, Toshikazu Ikuta, Jonathan C. Ipser, Asier Juaneda-Seguí, Hitomi Kitagawa, Gerd Kvale, Mafalda Machado-Sousa, Astrid Morer, Takashi Nakamae, Jin Narumoto, Joseph O'Neill, Sho Okawa, Eva Real, Veit Roessner, Joao R. Sato, Cinto Segalàs, Roseli G. Shavitt, Dick J. Veltman, Kei Yamada were missing from the author list indexed under the ENIGMA-OCD Working Group. Additionally, there was an error regarding Tokiko Yoshida’s name, where the first name and last name were written in the wrong order. The original article has been corrected.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bruin, W.B., Abe, Y., Alonso, P. et al. The functional connectome in obsessive-compulsive disorder: resting-state mega-analysis and machine learning classification for the ENIGMA-OCD consortium. Mol Psychiatry 28, 4307–4319 (2023). https://doi.org/10.1038/s41380-023-02077-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02077-0
This article is cited by
-
From compulsivity to compulsion: the neural basis of compulsive disorders
Nature Reviews Neuroscience (2024)
-
Topological Data Analysis Captures Task-Driven fMRI Profiles in Individual Participants: A Classification Pipeline Based on Persistence
Neuroinformatics (2023)