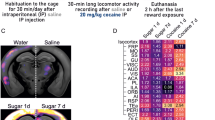
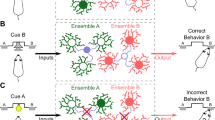
Abstract
Poorly regulated reward seeking is a central feature of substance use disorder. Recent research shows that rewarding drug-related experiences induce synchronous activation of a discrete number of neurons in the nucleus accumbens that are causally linked to reward-related contexts. Here we comprehensively characterize the specific ensemble of neurons built through experience that are linked to seeking behavior. We additionally address the question of whether or not addictive drugs usurp the neuronal networks recruited by natural rewards by evaluating cocaine- and sucrose-associated ensembles within the same mouse. We used FosCreERT2/+/Ai14 transgenic mice to tag cells activated by and potentially encoding cocaine and sucrose seeking. We tagged ~1% of neurons in the core subregion of the accumbens (NAcore) activated during cue-induced seeking for cocaine or sucrose. The majority of tagged cells in the seeking ensembles were D1-MSNs, and specifically activated during seeking, not during extinction or when mice remained in the home cage. To compare different reward-specific ensembles within the same mouse, we used a dual cocaine and sucrose self-administration protocol allowing reward-specific seeking. Using this model, we found ~70% distinction between the cells constituting the cocaine- compared to the sucrose-seeking ensemble. Establishing that cocaine recruits an ensemble of NAcore neurons largely distinct from neurons recruited into an ensemble coding for sucrose seeking suggest a finely tuned specificity of ensembles. The findings allow further exploration of the mechanisms that transform reward-based positive reinforcement into maladaptive drug seeking.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thorndike EL. Animal intelligence; experimental studies. New York: The Macmillan company; 1911.
American Psychiatric Association., American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th edn. Washington, D.C.: American Psychiatric Association; 2013, xliv, 947 p.pp.
Hadad NA, Knackstedt LA. Addicted to palatable foods: comparing the neurobiology of Bulimia Nervosa to that of drug addiction. Psychopharmacology. 2014;231:1897–912.
Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, et al. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14:743–54.
Whitaker LR, Hope BT. Chasing the addicted engram: identifying functional alterations in Fos-expressing neuronal ensembles that mediate drug-related learned behavior. Learn Mem. 2018;25:455–60.
Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–3.
Guenthner CJ, Miyamichi K, Yang HH, Heller HC, Luo L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron. 2013;78:773–84.
Ye L, Allen WE, Thompson KR, Tian Q, Hsueh B, Ramakrishnan C, et al. Wiring and molecular features of prefrontal ensembles representing distinct experiences. Cell. 2016;165:1776–88.
Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–73.
Warren BL, Kane L, Venniro M, Selvam P, Quintana-Feliciano R, Mendoza MP et al. Separate vmPFC ensembles control cocaine self-administration versus extinction in rats. J Neurosci. 2019;39:7394–407.
Warren BL, Mendoza MP, Cruz FC, Leao RM, Caprioli D, Rubio FJ, et al. Distinct Fos-expressing neuronal ensembles in the ventromedial prefrontal cortex mediate food reward and extinction memories. J Neurosci. 2016;36:6691–703.
Laque A, LDN G, Wagner GE, Nedelescu H, Carroll A, Watry D, et al. Anti-relapse neurons in the infralimbic cortex of rats drive relapse-suppression by drug omission cues. Nat Commun. 2019;10:3934.
Suto N, Laque A, De Ness GL, Wagner GE, Watry D, Kerr T et al. Distinct memory engrams in the infralimbic cortex of rats control opposing environmental actions on a learned behavior. Elife. 2016;5:e21920.
Zhou Y, Zhu H, Liu Z, Chen X, Su X, Ma C et al. A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci. 2019;22:1986–99.
Wall NR, Neumann PA, Beier KT, Mokhtari AK, Luo L, Malenka RC. Complementary genetic targeting and monosynaptic input mapping reveal recruitment and refinement of distributed corticostriatal ensembles by cocaine. Neuron. 2019;104:916–30.e5.
Mattson BJ, Koya E, Simmons DE, Mitchell TB, Berkow A, Crombag HS, et al. Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur J Neurosci. 2008;27:202–12.
Cameron CM, Carelli RM. Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. Eur J Neurosci. 2012;35:940–51.
Cruz FC, Babin KR, Leao RM, Goldart EM, Bossert JM, Shaham Y, et al. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci. 2014;34:7437–46.
Carelli RM. The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev. 2002;1:281–96.
Carelli RM, Deadwyler SA. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. J Neurosci. 1994;14:7735–46.
Carelli RM, Deadwyler SA. Cellular mechanisms underlying reinforcement-related processing in the nucleus accumbens: electrophysiological studies in behaving animals. Pharmacol Biochem Behav. 1997;57:495–504.
Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–66.
Pfarr S, Schaaf L, Reinert JK, Paul E, Herrmannsdorfer F, Rossmanith M, et al. Choice for drug or natural reward engages largely overlapping neuronal ensembles in the infralimbic prefrontal cortex. J Neurosci. 2018;38:3507–19.
Kane L, Venniro M, Quintana-Feliciano R, Madangopal R, Rubio FJ, Bossert JM et al. Fos-expressing neuronal ensemble in rat ventromedial prefrontal cortex encodes cocaine seeking but not food seeking in rats. Addict Biol. 2020;e12943. https://doi.org/10.1111/adb.12943. Online ahead of print.
Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, et al. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat Neurosci. 2019;22:753–61.
Manuela M, PP J, Joe H. Adaptation in patterns of c‐fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33.
Struthers WM, DuPriest A, Runyan J. Habituation reduces novelty-induced FOS expression in the striatum and cingulate cortex. Exp Brain Res. 2005;167:136–40.
Kovacs KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol. 2008;20:665–72.
McReynolds JR, Christianson JP, Blacktop JM, Mantsch JR. What does the Fos say? Using Fos-based approaches to understand the contribution of stress to substance use disorders. Neurobiol Stress. 2018;9:271–85.
Bariselli S, Fobbs WC, Creed MC, Kravitz AV. A competitive model for striatal action selection. Brain Res. 2019;1713:70–9.
Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Pena CJ, et al. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci USA. 2016;113:2726–31.
Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–8.
Heinsbroek JA, Neuhofer DN, Griffin WC 3rd, Siegel GS, Bobadilla AC, Kupchik YM, et al. Loss of plasticity in the D2-accumbens pallidal pathway promotes cocaine seeking. J Neurosci. 2017;37:757–67.
Roberts-Wolfe* D, Bobadilla* AC, Heinsbroek JA, Neuhofer D, Kalivas PW. Drug refraining and seeking potentiate synapses on distinct populations of accumbens medium spiny neurons. J Neurosci. 2018;38:7100–7.
Roberts-Wolfe DJ, Heinsbroek JA, Spencer SM, Bobadilla AC, Smith ACW, Gipson CD et al. Transient synaptic potentiation in nucleus accumbens shell during refraining from cocaine seeking. Addict Biol. 2020;e12943. https://doi.org/10.1111/adb.12943. Online ahead of print.
Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Addict Biol. 2020;25:e12759. https://doi.org/10.1111/adb.12759. Epub 2019 May 6.
Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, et al. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci: Off J Soc Neurosci. 2015;35:8232–44.
Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, et al. Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. J Neurosci: Off J Soc Neurosci. 2017;37:1014–27.
Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–2.
Creed M, Ntamati NR, Chandra R, Lobo MK, Luscher C. Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum. Neuron. 2016;92:214–26.
Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52.
Heinsbroek JA, Bobadilla AC, Dereschewitz E, Assali A, Chalhoub RM, Cowan CW, et al. Opposing regulation of cocaine seeking by glutamate and GABA neurons in the ventral pallidum. Cell Rep. 2020;30:2018–27 e2013.
Bobadilla AC, Garcia-Keller C, Heinsbroek JA, Scofield M, Chareunsouk V, Monforton C et al. Accumbens mechanisms for cued sucrose-seeking. Neuropsychopharmacology. 2017;42:2377–86.
Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, et al. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–2.
Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, Hope BT. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci. 2012;32:11600–9.
Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PloS One. 2007;2:e698.
Tunstall BJ, Kearns DN. Cocaine can generate a stronger conditioned reinforcer than food despite being a weaker primary reinforcer. Addict Biol. 2016;21:282–93.
Rubio FJ, Quintana-Feliciano R, Warren BL, Li X, Witonsky KFR, Soto Del Valle F et al. Prelimbic cortex is a common brain area activated during cue-induced reinstatement of cocaine and heroin seeking in a polydrug self-administration rat model. Eur J Neurosci. 2019;49:165–78.
Lynch WJ. Modeling the development of drug addiction in male and female animals. Pharmacol Biochem Behav. 2018;164:50–61.
Kuhn BN, Kalivas PW, Bobadilla A-C. Understanding addiction using animal models. Front Behav Neurosci. 2019;13:262. https://doi.org/10.3389/fnbeh.2019.00262. eCollection 2019.
Spencer S, Garcia-Keller C, Roberts-Wolfe D, Heinsbroek JA, Mulvaney M, Sorrell A, et al. Cocaine use reverses striatal plasticity produced during cocaine seeking. Biol Psychiatry. 2017;81:616–24.
Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–70.
Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol. 2013;23:99–106.
DeNardo LA, Liu CD, Allen WE, Adams EL, Friedmann D, Dadgar-Kiani E et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Preprint at https://www.biorxiv.org/content/10.1101/295238v1.full. 2018.
Bobadilla AC, Heinsbroek JA, Gipson CD, Griffin WC, Fowler CD, Kenny PJ, et al. Corticostriatal plasticity, neuronal ensembles, and regulation of drug-seeking behavior. Prog Brain Res. 2017;235:93–112.
Ziminski JJ, Sieburg MC, Margetts-Smith G, Crombag HS, Koya E. Regional differences in striatal neuronal ensemble excitability following cocaine and extinction memory retrieval in Fos-GFP mice. Neuropsychopharmacology. 2018;43:718–27.
Ziminski JJ, Hessler S, Margetts-Smith G, Sieburg MC, Crombag HS, Koya E. Changes in appetitive associative strength modulates nucleus accumbens, but not orbitofrontal cortex neuronal ensemble excitability. J Neurosci. 2017;37:3160–70.
Whitaker LR, Warren BL, Venniro M, Harte TC, McPherson KB, Beidel J, et al. Bidirectional modulation of intrinsic excitability in rat prelimbic cortex neuronal ensembles and non-ensembles after operant learning. J Neurosci. 2017;37:8845–56.
Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR, et al. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15:1556–62.
Whitaker LR, Carneiro de Oliveira PE, McPherson KB, Fallon RV, Planeta CS, Bonci A et al. Associative learning drives the formation of silent synapses in neuronal ensembles of the nucleus accumbens. Biol Psychiatry. 2016;80:246–56.
Wright WJ, Graziane NM, Neumann PA, Hamilton PJ, Cates HM, Fuerst L et al. Silent synapses dictate cocaine memory destabilization and reconsolidation. Nat Neurosci. 2020;23:32–46.
Josselyn SA, Tonegawa S. Memory engrams: recalling the past and imagining the future. Science. 2020;367:eaaw4325. https://doi.org/10.1126/science.aaw4325.
Tonegawa S, Liu X, Ramirez S, Redondo R. Memory engram cells have come of age. Science. 2020;367:eaaw4325. https://doi.org/10.1126/science.aaw4325.
Choi JH, Sim SE, Kim JI, Choi DI, Oh J, Ye S, et al. Interregional synaptic maps among engram cells underlie memory formation. Science. 2018;360:430–5.
Acknowledgements
The authors thank Drs. Chris Cowan and Makoto Taniguchi for kindly providing the initial cFos-TRAP mice, Jordan Hopkins and Dr. Ahlem Assali for their help optimizing the RNAscope protocol, and the members of the Kalivas lab for helpful comments on the manuscript. This work was supported by NIH DA046522 (A-CB), DA040004 (MDS), DA003906 (PWK), DA12513 (PWK).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bobadilla, AC., Dereschewitz, E., Vaccaro, L. et al. Cocaine and sucrose rewards recruit different seeking ensembles in the nucleus accumbens core. Mol Psychiatry 25, 3150–3163 (2020). https://doi.org/10.1038/s41380-020-00888-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-00888-z
This article is cited by
-
Whole-brain tracking of cocaine and sugar rewards processing
Translational Psychiatry (2023)
-
Net gain and loss: influence of natural rewards and drugs of abuse on perineuronal nets
Neuropsychopharmacology (2023)
-
External globus pallidus input to the dorsal striatum regulates habitual seeking behavior in male mice
Nature Communications (2023)
-
Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens
Molecular Psychiatry (2022)