Abstract
Objective
To determine costs of hospitalization associated with bronchopulmonary dysplasia (BPD) during the first year in very low birth weight infants.
Study design
Retrospective cohort study of California births from 2008 to 2011 linking birth certificate, discharge records, and clinical data from California Perinatal Quality Care Collaborative. Inclusion: birth weight 401–1500 g, gestational age < 30 weeks, inborn or transferred within 2 days, alive at 36 weeks corrected, and without major congenital anomalies. Outcomes included cost and length of stay of initial hospitalization and rehospitalizations.
Result
Out of 7998 eligible infants, 2696 (33.7%) developed BPD. Median hospitalization cost in the first year was $377,871 per infant with BPD compared with $175,836 per infant without BPD (adjusted cost ratio 1.54, 95% confidence interval (CI) 1.49–1.59). Infants with BPD also had longer length of stay and a higher likelihood of rehospitalization.
Conclusion
BPD is associated with substantial resource utilization. Prevention strategies could help conserve healthcare resources.
Similar content being viewed by others
Introduction
Very low birth weight infants (VLBW; birth weight < 1500 g) often require a significant amount of medical care after birth. Though the incidence of VLBW infants has remained stable in the United States at ~1.4% [1], infants with VLBW account for upward of 35% of all birth-related hospital costs [2, 3]. Studies have demonstrated substantial costs associated with prematurity-related morbidities, with bronchopulmonary dysplasia (BPD) being the most prevalent, affecting an estimated 22–45% of VLBW infants in the United States [4,5,6].
BPD may be the most expensive individual morbidity in VLBW infants, with reports of costs for initial hospitalization over two times higher than those without BPD [6, 7]. While the pathophysiology of BPD itself initially is lung related, infants with a diagnosis of BPD are at increased risk for late-onset sepsis, longer hospital length of stay (LOS), cerebral palsy, long-term neurodevelopmental difficulties, long-term pulmonary morbidities, as well as socioeconomic effects on the family and support system [8,9,10]. Infants with BPD are more than twice as likely to be readmitted to the hospital during the first year of life [11], and the health and financial impact of having BPD are profound and extend far beyond the initial birth hospitalization [9].
Though BPD rates have remained largely stagnant, there is evidence that quality improvement projects can successfully reduce BPD [12]. In addition, there are clinical interventions that have been shown to help decrease BPD, including vitamin A, surfactant, caffeine, postnatal corticosteroids, breast milk, and different ventilation strategies, including noninvasive ventilation [13,14,15,16]. Some of these interventions (e.g., breast milk [15] and caffeine [17]) are even cost saving. A comprehensive understanding of the economic burden of BPD more broadly and beyond the birth hospitalization will help inform future research on potential cost effectiveness of quality improvement efforts and interventions to reduce BPD. Thus, the purpose of this study is to quantify the incremental cost of BPD in the first year of life by comparing VLBW infants with BPD vs without BPD in a contemporary population-based cohort.
Methods
Participants
This was a retrospective cohort study using population-based data on births from January 2008 to December 2011 in the state of California linking birth certificate, hospital discharge records, and clinical data from the California Perinatal Quality Care Collaborative (CPQCC). CPQCC prospectively collects data on greater than 90% of VLBW infants receiving neonatal intensive care in the state of California. Data are collected by trained abstractors and data definitions are aligned and consistent with those of the Vermont Oxford Network (VON). Hospital discharge records, including information on charges, were obtained from California’s Office of Statewide Health Planning and Development (OSHPD). CPQCC database was linked to the OSHPD infant birth certificate and hospital discharge records by a probabilistic linkage algorithm. The study years were chosen due to availability of linked data for infants born from 2008 to 2011. This study was exempt by the Oregon Health & Science University Institutional Review Board and approved by the Stanford University Institutional Review Board. Due to the preexisting nature of the databases, informed consent was not required.
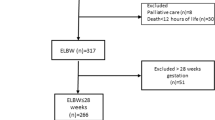
The sample cohort was limited to infants who met eligibility criteria for entry into the CPQCC database and included those with birth weight 401–1500 g and gestational age between 22 0/7 weeks and 29 6/7 weeks if they were inborn at a CPQCC hospital or transferred within 2 days after birth. Infants were excluded if they had major congenital anomalies or died prior to 36 weeks corrected gestational age (CGA) as this specific time point is required for the diagnosis of BPD (see “Exposure” section below).
Infants were excluded if their records could not be linked between CPQCC and OSHPD data (<2%). Furthermore, infants who were admitted to a Kaiser Hospital or Shriner’s Hospital for Children in Northern California were excluded from the cost analysis, as charges are not regularly reported in the OSHPD hospital discharge data for these institutions and thus total costs could not be estimated. We also excluded infants with records indicating the following outlier situations as they might suggest poor quality of data or clinical implausibility for VLBW infants: those with initial birth hospitalization that was 21 days or less, or if there was a large discrepancy in LOS (>18 days, which is equivalent to top 1%) between the two databases, or if adjusted cost per day was less than $500 or greater than $12,900 (consistent with previous research using these data sources and based on inflation adjusted 2014 US dollars [2, 3]).
Exposure
Comparisons were made between infants diagnosed with and without BPD. BPD was defined as oxygen use at 36 weeks CGA while inpatient, or if discharged home or transferred between 34 and 35 weeks CGA on oxygen, or on oxygen at time of discharge if oxygen use at 36 weeks CGA is unknown. This definition approximates the VON definition for BPD and was used in our analysis as CPQCC does not collect data on the physiologic definition of BPD. Infants who had unknown BPD status were excluded from the statistical analysis.
Outcome measures
Outcome measures included cost and LOS of the initial birth hospitalization and rehospitalizations within the first year of life. Only hospital facility costs were included in the analysis, while physician fees were not included due to lack of relevant data. Costs were estimated by converting charges for the infant from their hospital discharge records to cost using a cost-to-charge ratio (CCR) approach. Hospital-year-specific CCRs were derived from OSHPD Hospital Annual Financial Reporting data, reflecting the cost-to-charge relationship at a given hospital year. Costs were then adjusted by the consumer price index to reflect 2014 US dollars.
Statistical analyses
We first summarized the maternal and infant clinical characteristics of the study population. The association between BPD and maternal and infant clinical characteristics was examined using chi-square test. Outcome measures, i.e., LOS and cost of hospitalizations, were examined by BPD status and hospitalization type (birth/delivery hospitalization vs readmissions). Wilcoxon–Mann–Whitney test was used to test differences in the outcome measures between infants with BPD and without BPD. As a sensitivity analysis, we also compared hospitalization costs between the BPD and non-BPD group without any other comorbidities.
Generalized linear regression models (with log link and gamma distribution) were used to examine the association between BPD and hospitalization costs to account for skewness in cost distributions, while adjusting for other patient characteristics. Gamma distribution was selected based on the modified Park test, and log link function was chosen based on the Pregibon link test, and Box-Cox transformation. The regression model adjusted for a comprehensive set of clinical risk factors that were significantly different between infants with and without BPD. Based on significance in the bivariate association, sequential regression approach was used, building successive regression models in stages. We initially started from the demographic only model, clinical risk factors were then added to the next model. The third model added an interaction between GA and BPD. Ventilation use, surgery, and death were not included in order to estimate the effect of BPD as ventilation use and surgery can be considered an intermediary step in the causal pathway from the exposure variable to the outcome. The model fit was then evaluated using a likelihood ratio test and Akaike information criterion comparing different models. In the regression model, we adjusted for maternal age, race, parity, payer, fetal distress, chorioamnionitis, obstetrical bleeding, prolonged rupture of membranes, prenatal care, antenatal steroids, delivery mode, 5 min Apgar score, gestational age (weeks), multiple birth, sex, small for gestational age, necrotizing enterocolitis (NEC), severe retinopathy of prematurity (ROP), late-onset sepsis, severe intraventricular hemorrhage (IVH), BPD, and the interaction between BPD and gestational age. We did not adjust for birth weight as gestational age is highly correlated with birth weight and also more frequently used when counseling parents on the risks of prematurity. We also did not adjust for intrauterine growth restriction as small for gestational age is already included in the models. All statistical analyses were performed using SAS 9.4™ (Cary, NC) and Stata 14.0™ (College Station, TX).
Results
During 2008–2011, a total of 10,685 infants met inclusion criteria with available linked data between OSHPD and CPQCC. Of those infants, 7% were excluded due to either incomplete data or discrepancy in LOS and a further 13% excluded due to having received care at either Kaiser or Shriners Hospital for Northern California (see Supplementary Figure). The final analysis cohort included 7998 infants with 2696 (33.7%) meeting the definition for BPD. Comparison of these excluded infants with the remaining infants in our sample showed no meaningful differences in clinical characteristics (data not shown).
Infants with BPD were more likely to be born to women who were nulliparous, diagnosed with intrauterine growth restriction, had prolonged rupture of membranes, obstetrical bleeding, chorioamnionitis, or delivered via cesarean section (Table 1). Infants who developed BPD also were born at a significantly lower gestational age, had lower birth weight and lower 5 min Apgar scores, and were more likely male. They were less likely to be initially resuscitated with continuous positive airway pressure (CPAP) in the delivery room or receive nasal CPAP prior to intubation (Table 2). During the postnatal course, they were also more likely to be treated with surfactant and postnatal steroids. Infants with BPD had a higher rate of comorbidities, including NEC, ROP, IVH, late-onset sepsis, and any surgeries during birth hospitalization. In our cohort, 40% of infants with BPD were discharged home on oxygen support and 4.5% were discharged on a ventilator, compared with 2.7 and 1% for infants without BPD, respectively.
Infants with BPD had significantly longer unadjusted LOS for the birth hospitalization (median 103 days vs 66.5 days, p < 0.001) and cost more than twice as much (median $373,468 vs $173,287, p < 0.001) compared with infants without BPD (Table 3). The birth hospitalization for infants with BPD also had significantly higher unadjusted cost per day (median $3470 vs $2575 for BPD and no BPD, respectively). In addition, 25.1% of infants with BPD were rehospitalized in the first year of life compared with 16.2% of infants without BPD (p < 0.001). The total cost of hospitalization in the first year of life was primarily driven by the birth hospitalization (Table 3).
The unadjusted cost ratio for birth hospitalization and total hospitalization during the first year were 2.05 (95% CI: 1.99–2.11) and 2.06 (95% CI: 1.99–2.13), respectively (Table 4). After risk adjustment, the cost ratio of BPD was 1.52 (95% CI: 1.47–1.58) for the birth hospitalization, indicating that infants with BPD have 52% higher birth hospitalization costs than those without BPD on average (p < 0.001). Adjusted birth hospitalization cost was $331,769 (95% CI: $322,833–$340,951) for infants with BPD compared with $217,801 (95% CI: $214,548–$221,103) for infants without BPD. After risk adjustment, the cost ratio for BPD on the total hospitalization costs during the first year was 1.54 (95% CI: 1.49–1.59), and the adjusted total hospitalization costs were $340,446 (95% CI: $331,178–$349,973) and $221,636 (95% CI: $218,274–$225,050) for those infants with and without BPD, respectively (p < 0.001). Hospitalization costs in the first year were inversely related to gestational age and infants with BPD had significantly higher costs across all gestational ages (Fig. 1). The BPD group not only had a significantly higher proportion of infants with lower gestational age, the median total hospitalization costs for infants with BPD were also over $100,000 greater than infants without BPD at all gestational ages. The cost difference persisted despite adjusting for other factors known to influence BPD, including comorbidities that are more prevalent in patients with BPD.
As a sensitivity analysis, we further excluded infants with any comorbidities in our unadjusted analysis and found that infants without any morbidities had a median birth hospitalization cost of $152,511 (IQR = 104,450–219,643), while those with BPD but no other comorbidities had a median birth hospitalization cost of $252,851 (IQR = 184,591–369,542) (p < 0.0001).
Discussion
BPD is a significant morbidity of VLBW infants, affecting one-third of infants in the presented California cohort. The majority of first year hospitalization costs are associated with the birth hospitalization, despite a large proportion of the infants being readmitted post initial discharge home. BPD was associated with a 1.52-fold higher cost for initial hospitalization and a 1.54-fold higher costs for overall hospitalization in the first year of life compared with infants without BPD. Although healthcare costs are driven by gestational age and additional comorbidities, both of which are more prevalent in patients with BPD, the large costs differences persisted and remained statistically significant when adjusted for confounders and stratified by gestational age (albeit attenuated from initial estimates).
Our study reaffirms the significantly higher resource utilization in those with BPD compared with those without BPD. First, infants with BPD tend to have more comorbidities and complications compared to those without and this is likely reflected in the increased median LOS of over a month for the birth hospitalization (103 days vs 66.5 days). While LOS is an important contributor to the increased hospitalization cost of BPD, infants with BPD also used resources more intensely as reflected by their higher median cost per day compared with those infants without BPD ($3470/day vs $2575/day).
As the development of BPD itself is multifactorial and the causal pathway arguably contains many factors, we also did a subanalysis comparing VLBW infants without any comorbidities to VLBW infants with only BPD and no other comorbidities (as in, we excluded all infants with NEC, ROP, IVH, late-onset sepsis). In this subanalysis, infants with BPD alone still had an initial hospitalization cost of over $100,000 more than VLBW infants without any morbidities. Therefore, BPD is likely an independent risk factor for significantly higher hospitalization costs during the first year of life. Similar difference in costs between infants with and without BPD has been reported by Patel et al. who found median costs of birth hospitalization in an infant with BPD as $269,004 vs $117,078 in infants without BPD and by Russell et al. who found that BPD was the single costliest morbidity [7, 15]. These prior studies, however, have focused on birth hospitalization alone.
In this study, infants with BPD required rehospitalization at significantly higher rates and had a longer LOS as well as cost per day during the rehospitalization. While these numbers demonstrate the significant economic impact of having BPD, our study also confirms the burden of prematurity in general, with ~19% of all VLBW infants rehospitalized regardless of BPD diagnosis highlighting the significant healthcare utilization by premature infants beyond hospitalizations. Our rates of rehospitalization are lower than a previously reported study in infants <33 weeks GA born in 1995–1999 that found hospitalization rates to be 49% vs 23% in the first year of life for infants with BPD vs no BPD [11]. And while it is known that the burden of readmissions is high, there is still a difference in reported numbers of readmission in extremely premature infants ranging from 27 to 49% in the first 12–18 months of life [18]. These differences may be reflective of a change in admission criteria, cohort characteristics, or outpatient preventative measures over the past 20 years.
As a field, we are always looking for opportunities to reduce morbidity and mortality in the VLBW population, and thus reduce associated costs. With respect to BPD, the majority of studies have demonstrated that BPD rates have remained fairly stagnant during the past few decades [19]. While in search of the latest therapy to help reduce the rates of BPD, we must acknowledge there is variation in BPD rates across hospitals ranging from ~18 to 73% of VLBW infants, which suggests that there is an opportunity for improvement within the current available therapies [5]. The large variation in BPD rates in similar populations suggests that there are opportunities to directly study not only what might reduce the variation and rates of BPD, but perhaps also what cost-effective strategies may be employed to treat infants with BPD. The use of early CPAP for preterm infants is recommended by the American Academy of Pediatrics Committee on Fetus and Newborn, citing benefits from randomized controlled trials of decreased death or BPD, reduction in mechanical ventilation days, and reduction in postnatal corticosteroid therapy [20]. Our study did find that infants without BPD were significantly more likely to have any initial resuscitation with CPAP and a trial of CPAP prior to intubation (Table 2). While this may be due to some underlying patient differences, there have been a handful of published quality improvement projects that successfully showed reduction in BPD at their centers through potentially better practice bundles which often include initial CPAP use in the delivery room [12, 21,22,23]. Other potential strategies that may reduce BPD but were not captured in our study include optimization of respiratory support strategies (such as volume targeted ventilation), use of mother’s own milk, and routine administration of caffeine [24].
The best prevention strategy for BPD would be prevention of prematurity itself. However, the rates of VLBW infants have remained similar over the last several years, especially as neonatal intensive care unit (NICU) resuscitation and survival have increased. Furthermore, maternal morbidities appear to be increasing. In particular, the rate of maternal obesity has risen steeply with one out of four mothers in our cohort having a body mass index of 30 or greater. Implementing strategies to reduce BPD may be a pressing need, as maternal obesity is significantly associated with an increased risk of prematurity as well as development of BPD specifically [25,26,27]. Thus a focus on prevention strategies in and outside of the NICU is even more critical. With a national estimated incidence of 15,000 infants with BPD [10], a 10% reduction in rates of BPD could roughly reduce healthcare costs by $150 million US dollars annually in hospital facility costs associated with the first year of life alone. While some of those savings can be reinvested into quality improvement work related to BPD prevention, other can be used toward other programming and harm reduction as related to prematurity.
This study utilized a comprehensive population-based cohort with a large number of patients. The strengths include a combination of large sample size while leveraging two comprehensive databases that provide details of clinical information and costs. We must also acknowledge some limitations. First, we were only able to examine hospital facility costs for inpatient care and did not include physician fees, pharmaceutical fees, durable medical equipment (e.g., home oxygen, ventilator), costs for outpatient services (e.g., office visits), or other financial (e.g., transportation and productivity loss from work), and nonfinancial (e.g., psychological, time) impact on the family thereby largely underestimating actual cost difference between patients with and without BPD. The diagnosis of BPD was also based on a specific day of hospitalization, i.e., the very day the infant is 36 weeks CGA, and does not take into account what may happen afterward prior to discharge. The database also did not allow us to track infants who were transferred to long-term care facilities and account for associated costs of such inpatient chronic care. All estimated costs were converted from charge data and there are potential limitations in utilizing charge to cost conversions. In addition, we had to exclude 13% of eligible infants as they had received care at either Kaiser or Shriner’s Hospital in Northern California where charges were not routinely reported in our databases. However, our final cohort had a BPD rate of 33.7%, similar to previously published reports of VLBW infants [5, 28, 29]. Lastly, the generalizability of our findings may be limited to the perinatal health services in the state of California where regionalization and referral patterns may help optimize the appropriate birth of VLBW infants, which is known to be associated with decreased morbidity and mortality. In regions with less structured healthcare, the costs differences between infants with and without BPD can be further exacerbated.
Conclusion
BPD continues to be a significant morbidity in the VLBW population and is a significant contributor to healthcare costs during the first year of life in premature infants. Future research examining novel therapies or quality improvement initiatives to reduce rates of BPD can have a profound impact on healthcare costs.
References
Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2016. Natl Vital- Stat Rep. 2018;67:1–55.
Schmitt SK, Sneed L, Phibbs CS. Costs of newborn care in California: a population-based study. Pediatrics. 2006;117:154–60.
Phibbs CS, Schmitt SK, Cooper M, Gould JB, Lee HC, Profit J, et al. Birth hospitalization costs and days of care for mothers and neonates in California, 2009–2011. J Pediatr. 2019;204:118–25.e14
Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129:1019–26.
Lapcharoensap W, Gage SC, Kan P, Profit J, Shaw GM, Gould JB, et al. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr. 2015;169:e143676.
Johnson TJ, Patel AL, Jegier BJ, Engstrom JL, Meier PP. Cost of morbidities in very low birth weight infants. J Pediatr. 2013;162:243–9.e1.
Russell RB, Green NS, Steiner CA, Meikle S, Howse JL, Poschman K, et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120:e1–9.
Lapcharoensap W, Kan P, Powers RJ, Shaw GM, Stevenson DK, Gould JB, et al. The relationship of nosocomial infection reduction to changes in neonatal intensive care unit rates of bronchopulmonary dysplasia. J Pediatr. 2017;180:105–9.e1.
Lapcharoensap W, Lee HC, Nyberg A, Dukhovny D. Health care and societal costs of bronchopulmonary dysplasia. Neoreviews. 2018;19:e211–23.
Morrow CB, McGrath-Morrow SA, Collaco JM. Predictors of length of stay for initial hospitalization in infants with bronchopulmonary dysplasia. J Perinatol. 2018;38:1258–65.
Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr. 2004;144:799–803.
Payne NR, LaCorte M, Karna P, Chen S, Finkelstein M, Goldsmith JP, et al. Reduction of bronchopulmonary dysplasia after participation in the Breathsavers Group of the Vermont Oxford Network Neonatal Intensive Care Quality Improvement Collaborative. Pediatrics 2006;118 Suppl 2:S73–7.
Dumpa V, Bhandari V. Surfactant, steroids and non-invasive ventilation in the prevention of BPD. Semin Perinatol. 2018;42:444–52.
Klingenberg C, Wheeler KI, McCallion N, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst Rev. 2017;10:CD003666.
Patel AL, Johnson TJ, Robin B, Bigger HR, Buchanan A, Christian E, et al. Influence of own mother’s milk on bronchopulmonary dysplasia and costs. Arch Dis Child Fetal neonatal Ed. 2017;102:F256–61.
Jensen EA, Foglia EE, Schmidt B. Evidence-based pharmacologic therapies for prevention of bronchopulmonary dysplasia: application of the grading of recommendations assessment, development, and evaluation methodology. Clin Perinatol. 2015;42:755–79.
Dukhovny D, Lorch SA, Schmidt B, Doyle LW, Kok JH, Roberts RS, et al. Economic evaluation of caffeine for apnea of prematurity. Pediatrics. 2011;127:e146–55.
Underwood MA, Danielsen B, Gilbert WM. Cost, causes and rates of rehospitalization of preterm infants. J Perinatol. 2007;27:614–9.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–51.
Committee on F, Newborn, American Academy of P. Respiratory support in preterm infants at birth. Pediatrics. 2014;133:171–4.
Horbar JD, Rogowski J, Plsek PE, Delmore P, Edwards WH, Hocker J, et al. Collaborative quality improvement for neonatal intensive care. NIC/Q Project Investigators of the Vermont Oxford Network. Pediatrics. 2001;107:14–22.
Lapcharoensap W, Bennett MV, Powers RJ, Finer NN, Halamek LP, Gould JB, et al. Effects of delivery room quality improvement on premature infant outcomes. J Perinatol. 2017;37:349–54.
Lee HC, Powers RJ, Bennett MV, Finer NN, Halamek LP, Nisbet C, et al. Implementation methods for delivery room management: a quality improvement comparison study. Pediatrics. 2014;134:e1378–86.
Jensen EA. Prevention of bronchopulmonary dysplasia: a summary of evidence-based strategies. Neoreviews. 2019;20:e189–201.
Carmichael SL, Kan P, Gould JB, Stevenson DK, Shaw GM, Lee HC. Maternal prepregnancy body mass index and risk of bronchopulmonary dysplasia. Pediatr Res. 2017;82:8–13.
Gould JB, Mayo J, Shaw GM, Stevenson DK. March of Dimes Prematurity Research Center at Stanford University School of M. Swedish and American studies show that initiatives to decrease maternal obesity could play a key role in reducing preterm birth. Acta Paediatrica. 2014;103:586–91.
Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikstrom AK, et al. Maternal obesity and risk of preterm delivery. JAMA. 2013;309:2362–70.
Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–11.
Lagatta JM, Clark RH, Brousseau DC, Hoffmann RG, Spitzer AR. Varying patterns of home oxygen use in infants at 23-43 weeks’ gestation discharged from United States neonatal intensive care units. J Pediatr. 2013;163:976–82.e2.
Funding
The project was sponsored in part by grant number R01 HD087425 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver NICHD or National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lapcharoensap, W., Bennett, M.V., Xu, X. et al. Hospitalization costs associated with bronchopulmonary dysplasia in the first year of life. J Perinatol 40, 130–137 (2020). https://doi.org/10.1038/s41372-019-0548-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0548-x
This article is cited by
-
An epidemiological investigation of high-risk infants for Respiratory Syncytial Virus infections: a retrospective cohort study
Italian Journal of Pediatrics (2024)
-
Quality, outcome, and cost of care provided to very low birth weight infants in California
Journal of Perinatology (2024)
-
Incremental cost of premature birth – a public health care payer perspective from Hungary
BMC Health Services Research (2023)
-
Prematurity and BPD: what general pediatricians should know
European Journal of Pediatrics (2023)
-
Very-low-birth-weight infant short-term post-discharge outcomes: A retrospective study of specialized compared to standard care
Maternal and Child Health Journal (2023)