Abstract
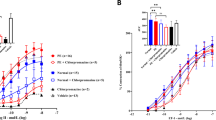
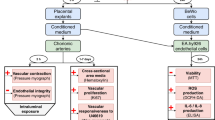
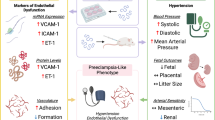
Endothelial cell dysfunction in pregnancy, which can be induced by placental factors, is the fundamental component of the pathogenesis of pre-eclampsia. The dysfunctional vascular endothelium disrupts the balance of vasodilatory and vasoconstrictive factors, resulting in increasing blood pressure. There is currently no effective treatment for pre-eclampsia and effective control of hypertension may reduce neonatal morbidity and mortality by prolonging gestation, especially in cases of early onset disease. To date methyldopa, labetalol, nifedipine and metoprolol are recommended for controlling blood pressure in pre-eclampsia. All of these drugs have different mechanisms of action. In this in vitro study we investigated whether different types of anti-hypertensive drugs could have different effects on improving maternal endothelial cell dysfunction. Endothelial cells (HMEC-1) were exposed to phorbol-12-myristate-13-acetate (PMA) or pre-eclamptic sera or extracellular vesicles (EVs) derived from pre-eclamptic placentae, in the presence of each of the studied anti-hypertensive drugs (methyldopa, labetalol, nifedipine and metoprolol) or placebo for 24 h. Endothelial cell-surface adhesion molecule (ICAM-1) and monocyte adhesion were measured. The expression of cell-face ICAM-1 by HMEC-1 cells and THP-1 monocyte adherent to HMEC-1 that were exposed to three separate well-known activators of endothelial cells in the presence of four anti-hypertensive drugs was significantly reduced regardless of the dose. However, the effect on the reduction of ICAM-1 expression and monocyte adhesion was not significantly different between the four medications. Our data suggest that the beneficial effect on improving endothelial cell function by these commonly prescribed anti-hypertensive drugs is seemingly independent of the anti-hypertensive mechanisms of the medication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99.
Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens. 1991;4:700–8.
Chen Q, Stone PR, McCowan LM, Chamley LW. Phagocytosis of necrotic but not apoptotic trophoblasts induces endothelial cell activation. Hypertension. 2006;47:116–21.
O’Brien M, Baczyk D, Kingdom JC. Endothelial dysfunction in severe preeclampsia is mediated by soluble factors, Rather than extracellular vesicles. Sci Rep. 2017;7:5887.
Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232:R27–R44.
Dzeshka MS, Shantsila A, Lip GY. Effects of aspirin on endothelial function and hypertension. Curr Hypertens Rep. 2016;18:83.
Varnier N, Brown MA, Reynolds M, Pettit F, Davis G, Mangos G, et al. Indications for delivery in pre-eclampsia. Pregnancy Hypertens. 2018;11:12–17.
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310.
MacCarthy EP, Bloomfield SS. Labetalol: a review of its pharmacology, pharmacokinetics, clinical uses and adverse effects. Pharmacotherapy. 1983;3:193–219.
Ueng KC, Lin MC, Chan KC, Lin CS. Nifedipine gastrointestinal therapeutic system: an overview of its antiatherosclerotic effects. Expert Opin Drug Metab Toxicol. 2007;3:769–80.
Flenady V, Wojcieszek AM, Papatsonis DN, Stock OM, Murray L, Jardine LA, et al. Calcium channel blockers for inhibiting preterm labour and birth. Cochrane Database Syst Rev. 2014;6:CD002255.
Morris J, Dunham A. Metoprolol. StatPearls. StatPearls Publishing LLC.; 2020.
Easterling TR. Pharmacological management of hypertension in pregnancy. Semin Perinatol. 2014;38:487–95.
Duan L, Ng A, Chen W, Spencer HT, Nguyen J, Shen AY, et al. beta-blocker exposure in pregnancy and risk of fetal cardiac anomalies. JAMA Intern Med. 2017;177:885–7.
Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA. 2000;283:1295–302.
Biswas TK. Endothelium, atherosclerosis and calcium channel blockers. J Indian Med Assoc. 2003;101:428–31.
Xu B, Charlton F, Makris A, Hennessy A. Antihypertensive drugs methyldopa, labetalol, hydralazine, and clonidine improve trophoblast interaction with endothelial cellular networks in vitro. J Hypertens. 2014;32:1075–83.
Chen Q, Guo F, Liu S, Xiao J, Wang C, Snowise S, et al. Calcium channel blockers prevent endothelial cell activation in response to necrotic trophoblast debris: possible relevance to pre-eclampsia. Cardiovasc Res. 2012;96:484–93.
Okesene-Gafa KAM, Li M, McKinlay CJD, Taylor RS, Rush EC, Wall CR, et al. Effect of antenatal dietary interventions in maternal obesity on pregnancy weight-gain and birthweight: Healthy Mums and Babies (HUMBA) randomized trial. Am J Obstet Gynecol. 2019;221:152 e1–152 e13.
Abumaree M, Stone P, Chamley L. An in vitro model of human placental trophoblast deportation/shedding. Mol Hum Reprod. 2006;12:687–92.
Tong M, Kleffmann T, Pradhan S, Johansson CL, DeSousa J, Stone PR, et al. Proteomic characterization of macro-, micro- and nano-extracellular vesicles derived from the same first trimester placenta: relevance for feto-maternal communication. Hum Reprod. 2016;31:687–99.
Berger B, Bachmann F, Duthaler U, Krähenbühl S, Haschke M. Cytochrome P450 enzymes involved in metoprolol metabolism and use of metoprolol as a CYP2D6 phenotyping probe drug. Front Pharmacol. 2018;9:774–774.
Chen Q, Stone P, Woon S, Ching L, Hung S, McCowan L, et al. Antiphospholipid antibodies bind to activated but not resting endothelial cells: is an independent triggering event required to induce antiphospholipid antibody-mediated disease? Thrombosis Res. 2004;114:101–11.
Roberts JM, Edep ME, Goldfien A, Taylor RN. Sera from preeclamptic women specifically activate human umbilical vein endothelial cells in vitro: morphological and biochemical evidence. Am J Reprod Immunol. 1992;27:101–8.
Xiao X, Xiao F, Zhao M, Tong M, Wise MR, Stone PR, et al. Treating normal early gestation placentae with preeclamptic sera produces extracellular micro and nano vesicles that activate endothelial cells. J Reprod Immunol. 2017;120:34–41.
Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83.
Tsatsaris V, Muller F, Maillard F, Delattre M, Guibourdenche J, Dreux S, et al. OS039. Early prediction of preeclampsia with maternal parameters,SVEGF-R1, PLGF, Inhibin-A and PAPP-A in general population: results from the MSPE study. Pregnancy Hypertens. 2012;2:197–8.
Gangooly S, Muttukrishna S, Jauniaux E. In-vitro study of the effect of anti-hypertensive drugs on placental hormones and angiogenic proteins synthesis in pre-eclampsia. PLoS ONE. 2014;9:e107644.
Sandoo A, van Zanten JJCSV, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010;4:302–12.
Panza JA, Quyyumi AA, Brush JE Jr., Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–7.
Chen Q, Guo F, Liu SR, Xiao JP, Wang C, Snowise S, et al. Calcium channel blockers prevent endothelial cell activation in response to necrotic trophoblast debris: possible relevance to pre-eclampsia. Cardiovasc Res. 2012;96:484–93.
Verhaar MC, Honing ML, van Dam T, Zwart M, Koomans HA, Kastelein JJ, et al. Nifedipine improves endothelial function in hypercholesterolemia, independently of an effect on blood pressure or plasma lipids. Cardiovasc Res. 1999;42:752–60.
Yamasaki K, Aoki M, Makino H, Hashiya N, Shimizu H, Ohishi M, et al. Effect of nifedipine on endothelial function in normotensive smokers: potential contribution of increase in circulating hepatocyte growth factor. J Hum Hypertens. 2004;18:701–5.
Sugiura T, Kondo T, Kureishi-Bando Y, Numaguchi Y, Yoshida O, Dohi Y, et al. Nifedipine improves endothelial function: role of endothelial progenitor cells. Hypertension. 2008;52:491–8.
Majidinia M, Rasmi Y, Khadem Ansari MH, Seyed-Mohammadzad M, Saboory E, Shirpoor A. Metoprolol improves endothelial function in patients with cardiac syndrome X. Iran J Pharm Res. 2016;15:561–6.
Wang Y, Walsh SW. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. J Soc Gynecol Invest. 1996;3:179–84.
Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol. 1998;16:93–104.
Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222:222–35.
Wang Y, Liu C, He X, Li Y, Zou Y. Effects of metoprolol, methyldopa, and nifedipine on endothelial progenitor cells in patients with gestational hypertension and preeclampsia. Clin Exp Pharmacol Physiol. 2019;46:302–12.
Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–86.
Xu B, Bobek G, Makris A, Hennessy A. Antihypertensive methyldopa, labetalol, hydralazine, and clonidine reversed tumour necrosis factor-alpha inhibited endothelial nitric oxide synthase expression in endothelial-trophoblast cellular networks. Clin Exp Pharmacol Physiol. 2017;44:421–7.
Acknowledgements
We would like to thank women with pre-eclampsia who donated blood samples and placentae.
Funding
This study was supported by a School of Medicine Performance based research fund (PBRF) funding from The University of Auckland, New Zealand and A+ trust from Auckland City Hospital, New Zealand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Woolston, E., Tang, Y., Azizi, S. et al. Comparison of the effects on maternal endothelial cell activation: an in vitro study of anti-hypertensive drugs clinically used in pre-eclampsia. J Hum Hypertens 36, 192–200 (2022). https://doi.org/10.1038/s41371-021-00497-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-021-00497-5
This article is cited by
-
Beta-Blockers in Pregnancy: Clinical Update
Current Hypertension Reports (2023)