Abstract
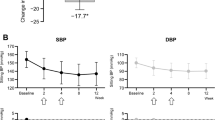
The steroidal mineralocorticoid receptor (MR) antagonists, spironolactone and eplerenone, decrease blood pressure, and attenuate the progression of chronic kidney disease (CKD). However, their use is limited by the fear of inducing hyperkalemia, gynecomastia, impotence, and amenorrhea. Esaxerenone is a novel nonsteroidal MR blocker (MRB) that has been recently developed. In vitro studies have revealed that esaxerenone has a high potency and selectivity for MR compared with spironolactone and eplerenone. Further studies have shown that esaxerenone elicits a strong blood pressure-lowering effect in hypertensive animals. Following the results from phase III clinical trials that esaxerenone is an effective and well-tolerated MRB in Japanese hypertensive patients, esaxerenone became clinically available in Japan from May 2019 for hypertensive patients. Thus, esaxerenone is a promising treatment option for patients with hypertension. In addition, both preclinical studies and phase II clinical trials have shown that esaxerenone elicits renoprotection independent of its antihypertensive effect. Recently, a phase III clinical trial (ESAX-DN study) has also demonstrated the safety and efficacy of esaxerenone in patients with type 2 diabetes and microalbuminuria. These data support future clinical development of esaxerenone for the treatment of renal disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Nishiyama A, Kobori H. Independent regulation of renin-angiotensin-aldosterone system in the kidney. Clin Exp Nephrol. 2018;22:1231–9.
Nishiyama A. Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens Res. 2019;42:293–300.
Fuller PJ, Yao Y, Yang J, Young MJ. Mechanisms of ligand specificity of the mineralocorticoid receptor. J Endocrinol. 2012;213:15–24.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17.
Samuel JL, Delcayre C. Heart failure: aldosterone antagonists are underused by clinicians. Nat Rev Cardiol. 2010;7:125–7.
Weinberger MH, Roniker B, Krause SL, Weiss RJ. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens. 2002;15:709–16.
Vukadinovic D, Lavall D, Vukadinovic AN, Pitt B, Wagenpfeil S, Bohm M. True rate of mineralocorticoid receptor antagonists-related hyperkalemia in placebo-controlled trials: a meta-analysis. Am Heart J. 2017;188:99–108.
Barfacker L, Kuhl A, Hillisch A, Grosser R, Figueroa-Perez S, Heckroth H, et al. Discovery of BAY 94-8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem. 2012;7:1385–403.
Arai K, Homma T, Morikawa Y, Ubukata N, Tsuruoka H, Aoki K, et al. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharmacol. 2015;761:226–34.
Bramlage P, Swift SL, Thoenes M, Minguet J, Ferrero C, Schmieder RE. Non-steroidal mineralocorticoid receptor antagonism for the treatment of cardiovascular and renal disease. Eur J Heart Fail. 2016;18:28–37.
Pei H, Wang W, Zhao D, Wang L, Su GH, Zhao Z. The use of a novel non-steroidal mineralocorticoid receptor antagonist finerenone for the treatment of chronic heart failure: a systematic review and meta-analysis. Medicine. 2018;97:e0254.
Lainscak M, Pelliccia F, Rosano G, Vitale C, Schiariti M, Greco C, et al. Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone. Int J Cardiol. 2015;200:25–9.
Piotrowski DW. Mineralocorticoid receptor antagonists for the treatment of hypertension and diabetic nephropathy. J Med Chem. 2012;55:7957–66.
Duggan S. Esaxerenone: first global approval. Drugs. 2019;79:477–81.
Takahashi M, Ubukata O, Homma T, Asoh Y, Honzumi M, Hayashi N, et al. Crystal structure of the mineralocorticoid receptor ligand-binding domain in complex with a potent and selective nonsteroidal blocker, esaxerenone (CS-3150). FEBS Lett. 2020. https://doi.org/10.1002/1873-3468.13746.
Bamberg K, Johansson U, Edman K, William-Olsson L, Myhre S, Gunnarsson A, et al. Preclinical pharmacology of AZD9977: a novel mineralocorticoid receptor modulator separating organ protection from effects on electrolyte excretion. PLoS ONE. 2018;13:e0193380.
Yamada M, Takei M, Suzuki E, Takakusa H, Kotsuma M, Washio T, et al. Pharmacokinetics, distribution, and disposition of esaxerenone, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist, in rats and monkeys. Xenobiotica. 2017;47:1090–103.
Yamada M, Mendell J, Takakusa H, Shimizu T, Ando O. Pharmacokinetics, metabolism, and excretion of [(14)C]esaxerenone, a novel mineralocorticoid receptor blocker in humans. Drug Metab Dispos. 2019;47:340–9.
Kurata A, Furuie H, Ishizuka T, Nakatsu T, Shimizu T, Kato M, et al. Absolute bioavailability of esaxerenone and food effects on its pharmacokinetics after a single oral dose in healthy japanese subjects: an open-label crossover study. Adv Ther. 2019;36:1618–27.
Ito S, Itoh H, Rakugi H. Efficacy and safety of esaxerenone (CS-3150) for the treatment of essential hypertension: a phase 2 randomized, placebo-controlled, double-blind study. J Hum Hypertens. 2019;33:542–51.
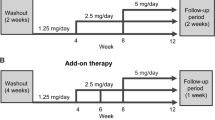
Rakugi H, Ito S, Itoh H, Okuda Y, Yamakawa S. Long-term phase 3 study of esaxerenone as mono or combination therapy with other antihypertensive drugs in patients with essential hypertension. Hypertens Res. 2019;42:1932–41.
Itoh H, Ito S, Rakugi H, Okuda Y, Nishioka S. Efficacy and safety of dosage-escalation of low-dosage esaxerenone added to a RAS inhibitor in hypertensive patients with type 2 diabetes and albuminuria: a single-arm, open-label study. Hypertens Res. 2019;42:1572–81.
Ito S, Shikata K, Nangaku M, Okuda Y, Sawanobori T. Efficacy and safety of esaxerenone (CS-3150) for the treatment of type 2 diabetes with microalbuminuria: a randomized, double-blind, placebo-controlled, phase II trial. Clin J Am Soc Nephrol. 2019;14:1161–72.
Kurata A, Yoshida T, Inoue M, Ishizuka T, Nakatsu T, Shimizu T, et al. Pharmacokinetics and safety of single-dose esaxerenone in japanese subjects with mild to moderate hepatic impairment. Adv Ther. 2019. https://doi.org/10.1007/s12325-019-01121-2.
Kato M, Furuie H, Shimizu T, Miyazaki A, Kobayashi F, Ishizuka H. Single- and multiple-dose escalation study to assess pharmacokinetics, pharmacodynamics and safety of oral esaxerenone in healthy Japanese subjects. Br J Clin Pharmacol. 2018;84:1821–9.
Brown NJ. Eplerenone: cardiovascular protection. Circulation. 2003;107:2512–8.
Arai K, Morikawa Y, Ubukata N, Tsuruoka H, Homma T. CS-3150, a novel nonsteroidal mineralocorticoid receptor antagonist, shows preventive and therapeutic effects on renal injury in deoxycorticosterone acetate/salt-induced hypertensive rats. J Pharmacol Exp Ther. 2016;358:548–57.
Li L, Guan Y, Kobori H, Morishita A, Kobara H, Masaki T, et al. Effects of the novel nonsteroidal mineralocorticoid receptor blocker, esaxerenone (CS-3150), on blood pressure and urinary angiotensinogen in low-renin Dahl salt-sensitive hypertensive rats. Hypertens Res. 2019;42:769–78.
Arai K, Tsuruoka H, Homma T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol. 2015;769:266–73.
Bhuiyan AS, Rafiq K, Kobara H, Masaki T, Nakano D, Nishiyama A. Effect of a novel nonsteroidal selective mineralocorticoid receptor antagonist, esaxerenone (CS-3150), on blood pressure and renal injury in high salt-treated type 2 diabetic mice. Hypertens Res. 2019;42:892–902.
Gorini S, Kim SK, Infante M, Mammi C, La Vignera S, Fabbri A, et al. Role of aldosterone and mineralocorticoid receptor in cardiovascular aging. Front Endocrinol. 2019;10:584.
Funder JW. Aldosterone and mineralocorticoid receptors-497 physiology and pathophysiology. Int J Mol Sci. 2017;18:1032.
Kobayashi N, Yoshida K, Nakano S, Ohno T, Honda T, Tsubokou Y, et al. Cardioprotective mechanisms of eplerenone on cardiac performance and remodeling in failing rat hearts. Hypertension. 2006;47:671–9.
Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Barfacker L, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64:69–78.
Grune J, Benz V, Brix S, Salatzki J, Blumrich A, Hoft B, et al. Steroidal and nonsteroidal mineralocorticoid receptor antagonists cause differential cardiac gene expression in pressure overload-induced cardiac hypertrophy. J Cardiovasc Pharmacol. 2016;67:402–11.
Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN study). Hypertension. 2020;75:51–8.
Okamura K, Matsushima M, Yamamoto F, Takamiya Y, Okuda T, Shirai K, et al. A patient with bilateral primary aldosteronism refractory to oral eplerenone who responded to esaxerenone with increased renin activity. Am J Case Rep. 2020;21:e920615.
Kolkhof P, Barfacker L. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. 2017;234:T125–t140.
Yang J, Young MJ. Mineralocorticoid receptor antagonists-pharmacodynamics and pharmacokinetic differences. Curr Opin Pharmacol. 2016;27:78–85.
Carone L, Oxberry SG, Twycross R, Charlesworth S, Mihalyo M, Wilcock A. Spironolactone. J Pain Symptom Manag. 2017;53:288–92.
Acknowledgements
We thank Jodi Smith, Ph.D., from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AN has received speaking honoraria from Taisho, Tanabe-Mitsubishi, Boehringer Ingelheim, Daiichi-Sankyo, and has received research funds from Daiichi-Sankyo, Boehringer Ingelheim, Bayer and Taisho. The funders had no role in the preparation of this paper. This study was also supported in part by the Salt Sciences Foundation (to AN).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wan, N., Rahman, A. & Nishiyama, A. Esaxerenone, a novel nonsteroidal mineralocorticoid receptor blocker (MRB) in hypertension and chronic kidney disease. J Hum Hypertens 35, 148–156 (2021). https://doi.org/10.1038/s41371-020-0377-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-0377-6
This article is cited by
-
Efficacy and Safety of Esaxerenone in Hypertensive Patients with Left Ventricular Hypertrophy (ESES-LVH) Study: A Multicenter, Open-Label, Prospective, Interventional Study
Advances in Therapy (2024)
-
2-phenylacetamide Separated from the seed of Lepidium apetalum Willd. inhibited renal fibrosis via MAPK pathway mediated RAAS and oxidative stress in SHR Rats
BMC Complementary Medicine and Therapies (2023)
-
Nighttime home blood pressure lowering effect of esaxerenone in patients with uncontrolled nocturnal hypertension: the EARLY-NH study
Hypertension Research (2023)
-
Factors Associated with the Antihypertensive Effect of Esaxerenone and Serum Potassium Elevation: A Pooled Analysis of Seven Phase III Studies
Advances in Therapy (2023)
-
Mineralocorticoid receptor antagonists for cardioprotection in chronic kidney disease: a step into the future
Journal of Human Hypertension (2022)