Abstract
In the United States each year, more than 300,000 infants are admitted to neonatal intensive care units (NICU) where they are exposed to a chemical-intensive hospital environment during a developmentally vulnerable period. Although multiple studies have demonstrated elevated phthalate biomarkers in NICU patients, specific sources of NICU-based phthalate exposure have not been identified.
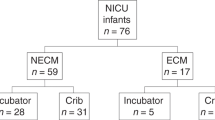
In this study, premature newborns with birth weight <1500 g were recruited to participate in a prospective environmental health cohort during the NICU hospitalization. Exposure to specific NICU equipment was recorded daily during the NICU hospitalization. One hundred forty-nine urine specimens from 71 infants were analyzed for phthalate metabolites using high-performance liquid chromatography/tandem mass spectrometry.
In initial analyses, exposure to medical equipment was directly related to phthalate levels, with DEHP biomarkers 95–132% higher for infants exposed to specific medical equipment types compared to those without that equipment exposure (p < 0.001–0.023). This association was mirrored for clinically relevant phthalate mixtures whether composed of DEHP metabolites or not (p = 0.002–0.007). In models accounting for concurrent equipment use, exposure to respiratory support was associated with DEHP biomarkers 50–136% higher in exposed compared to unexposed infants (p = 0.007–0.036). Phthalate mixtures clinically relevant to neurobehavioral development were significantly associated with non-invasive respiratory support (p = 0.008–0.026). Feeding supplies and intravenous lines were not significantly associated with clinically important phthalate mixtures.
Respiratory support equipment may be a significant and clinically relevant NICU source of phthalate exposure. Although manufacturers have altered feeding and intravenous supplies to reduce DEHP exposure, other sources of exposure to common and clinically impactful phthalates persist in the NICU.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–7.
Meredith RM. Sensitive and critical periods during neurotypical and aberrant neurodevelopment: a framework for neurodevelopmental disorders. Neurosci Biobehav Rev. 2015;50:180–8.
Mueller CA, Eme J, Burggren WW, Roghair RD, Rundle SD. Challenges and opportunities in developmental integrative physiology. Comp Biochem Physiol A Mol Integr Physiol. 2015;184:113–24.
Stroustrup A, Teitelbaum SL, Aschner JL. The value of preterm infant environmental health cohorts: the canary in the coal mine. JAMA Pediatr. 2017;171:1139–40.
Kraft AD, Aschner M, Cory-Slechta DA, Bilbo SD, Caudle WM, Makris SL. Unmasking silent neurotoxicity following developmental exposure to environmental toxicants. Neurotoxicol Teratol. 2016;55:38–44.
Mundy WR, Padilla S, Breier JM, Crofton KM, Gilbert ME, Herr DW, et al. Expanding the test set: chemicals with potential to disrupt mammalian brain development. Neurotoxicol Teratol. 2015;52(Pt A):25–35.
Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–28.
Scharf RJ, Stroustrup A, Conaway MR, DeBoer MD. Growth and development in children born very low birth weight. Arch Dis Child Fetal Neonatal Ed. 2016;101:F433–8.
Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123:1485–92.
Treyvaud K, Ure A, Doyle LW, Lee KJ, Rogers CE, Kidokoro H, et al. Psychiatric outcomes at age seven for very preterm children: rates and predictors. J Child Psychol Psychiatry. 2013;54:772–9.
Andrews B, Lagatta J, Chu A, Plesha-Troyke S, Schreiber M, Lantos J, et al. The non-impact of gestational age on neuro-developmental outcome for ventilated survivors born at 23–28 weeks gestation. Acta Paediatr. 2012;101:574–8.
Conrad AL, Richman L, Lindgren S, Nopoulos P. Biological and environmental predictors of behavioral sequelae in children born preterm. Pediatrics. 2010;125:e83–9.
Calafat AM, Needham LL, Silva MJ, Lambert G. Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics. 2004;113:e429–34.
Green R, Hauser R, Calafat AM, Weuve J, Schettler T, Ringer S, et al. Use of di(2-ethylhexyl) phthalate-containing medical products and urinary levels of mono(2-ethylhexyl) phthalate in neonatal intensive care unit infants. Environ Health Perspect. 2005;113:1222–5.
Latini G, De Felice C, Del Vecchio A, Barducci A, Ferri M, Chiellini F. Di-(2-ethylhexyl)phthalate leakage and color changes in endotracheal tubes after application in high-risk newborns. Neonatology. 2009;95:317–23.
Latini G, Ferri M, Chiellini F. Materials degradation in PVC medical devices, DEHP leaching and neonatal outcomes. Curr Med Chem. 2010;17:2979–89.
Weuve J, Sanchez BN, Calafat AM, Schettler T, Green RA, Hu H, et al. Exposure to phthalates in neonatal intensive care unit infants: urinary concentrations of monoesters and oxidative metabolites. Environ Health Perspect. 2006;114:1424–31.
Department of Health and Human Services, Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA; 2009.https://www.cdc.gov/exposurereport/index.html.
Calafat AM, Ye X, Silva MJ, Kuklenyik Z, Needham LL. Human exposure assessment to environmental chemicals using biomonitoring. Int J Androl. 2006;29:166–71. discussion 81–5.
Ventrice P, Ventrice D, Russo E, De Sarro G. Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environ Toxicol Pharmacol. 2013;36:88–96.
Anderson WA, Castle L, Hird S, Jeffery J, Scotter MJ. A twenty-volunteer study using deuterium labelling to determine the kinetics and fractional excretion of primary and secondary urinary metabolites of di-2-ethylhexylphthalate and di-iso-nonylphthalate. Food Chem Toxicol. 2011;49:2022–9.
Kessler W, Numtip W, Volkel W, Seckin E, Csanady GA, Putz C, et al. Kinetics of di(2-ethylhexyl) phthalate (DEHP) and mono(2-ethylhexyl) phthalate in blood and of DEHP metabolites in urine of male volunteers after single ingestion of ring-deuterated DEHP. Toxicol Appl Pharmacol. 2012;264:284–91.
Koch HM, Angerer J. Di-iso-nonylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP. Int J Hyg Environ Health. 2007;210:9–19.
Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79:367–76.
Leng G, Koch HM, Gries W, Schutze A, Langsch A, Bruning T, et al. Urinary metabolite excretion after oral dosage of bis(2-propylheptyl) phthalate (DPHP) to five male volunteers--characterization of suitable biomarkers for human biomonitoring. Toxicol Lett. 2014;231:282–8.
Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, et al. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009;30:522–8.
Ferguson KK, Peterson KE, Lee JM, Mercado-Garcia A, Blank-Goldenberg C, Tellez-Rojo MM, et al. Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reprod Toxicol. 2014;47:70–6.
Tellez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, Hu H, et al. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci Total Environ. 2013;461–2:386–90.
Cho SC, Bhang SY, Hong YC, Shin MS, Kim BN, Kim JW, et al. Relationship between environmental phthalate exposure and the intelligence of school-age children. Environ Health Perspect. 2010;118:1027–32.
Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, et al. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children’s Environmental Health (MOCEH) study. Environ Health Perspect. 2011;119:1495–500.
Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–84.
Ejaredar M, Nyanza EC, Ten Eycke K, Dewey D. Phthalate exposure and childrens neurodevelopment: a systematic review. Environ Res. 2015;142:51–60.
Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol. 2011;33:558–66.
Park S, Kim BN, Cho SC, Kim Y, Kim JW, Lee JY, et al. Association between urine phthalate levels and poor attentional performance in children with attention-deficit hyperactivity disorder with evidence of dopamine gene-phthalate interaction. Int J Environ Res Public Health. 2014;11:6743–56.
Testa C, Nuti F, Hayek J, De Felice C, Chelli M, Rovero P, et al. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. ASN Neuro. 2012;4:223–9.
Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, et al. Persistent associations between maternal prenatal exposure to phthalates on child IQ at age 7 years. PLoS ONE. 2014;9:e114003.
Fischer CJ, Bickle Graz M, Muehlethaler V, Palmero D, Tolsa JF. Phthalates in the NICU: is it safe? J Paediatr Child Health. 2013;49:E413–9.
Mallow EB, Fox MA. Phthalates and critically ill neonates: device-related exposures and non-endocrine toxic risks. J Perinatol. 2014;34:892–7.
Santos J, Pearce SE, Stroustrup A. Impact of hospital-based environmental exposures on neurodevelopmental outcomes of preterm infants. Curr Opin Pediatr. 2015;27:254–60.
Stroustrup A, Bragg JB, Andra S, Curtin P, Spear EA, Sison DB, et al. Neonatal intensive care unit phthalate exposure and preterm infant neurobehavioral performance. PLoS ONE. 2018;13:e0193835.
Boukydis CF, Bigsby R, Lester BM. Clinical use of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113(3 Pt 2):679–89.
Lester BM, Tronick EZ. History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113(3 Pt 2):634–40.
Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;113(3 Pt 2):641–67.
Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, et al. Summary statistics of Neonatal Intensive Care Unit Network Neurobehavioral Scale scores from the maternal lifestyle study: a quasinormative sample. Pediatrics. 2004;113(3 Pt 2):668–75.
Tronick E, Lester BM. Grandchild of the NBAS: the NICU Network Neurobehavioral Scale (NNNS): a review of the research using the NNNS. J Child Adolesc Psychiatr Nurs. 2013;26:193–203.
Tronick EZ, Olson K, Rosenberg R, Bohne L, Lu J, Lester BM. Normative neurobehavioral performance of healthy infants on the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113(3 Pt 2):676–8.
Liu J, Bann C, Lester B, Tronick E, Das A, Lagasse L, et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics. 2010;125:e90–8.
Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110:1182–92.
Montirosso R, Del Prete A, Bellu R, Tronick E, Borgatti R. Neonatal Adequate Care for Quality of Life Study G. Level of NICU quality of developmental care and neurobehavioral performance in very preterm infants. Pediatrics. 2012;129:e1129–37.
Smith JR, McGrath J, Brotto M, Inder T. A randomized-controlled trial pilot study examining the neurodevelopmental effects of a 5-week M Technique intervention on very preterm infants. Adv Neonatal Care. 2014;14:187–200.
Sampson J, de Korte D. DEHP-plasticised PVC: relevance to blood services. Transfus Med. 2011;21:73–83.
Carrico C, Gennings C, Wheeler D, Factor-Litvak P. Characterization of a weighted quartile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat. 2014;20:100–20.
Lester BM, Andreozzi-Fontaine L, Tronick E, Bigsby R. J Vis Exp. 2014 Aug 25;(90). Assessment and evaluation of the high risk neonate: the NICU Network Neurobehavioral Scale. https://doi.org/10.3791/3368. PMID:25177897. PMCID: PMC4828009.
Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22:745.
Parry G, Tucker J, Tarnow-Mordi W, Group UKNSSC. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;361:1789–91.
Van Vliet ED, Reitano EM, Chhabra JS, Bergen GP, Whyatt RM. A review of alternatives to di (2-ethylhexyl) phthalate-containing medical devices in the neonatal intensive care unit. J Perinatol. 2011;31:551–60.
Su PH, Chang YZ, Chang HP, Wang SL, Haung HI, Huang PC, et al. Exposure to di(2-ethylhexyl) phthalate in premature neonates in a neonatal intensive care unit in Taiwan. Pediatr Crit Care Med. 2012;13:671–7.
Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect. 2012;120:290–5.
Demirel A, Coban A, Yildirim S, Dogan C, Sanci R, Ince Z. Hidden toxicity in neonatal intensive care units: phthalate exposure in very low birth weight infants. J Clin Res Pediatr Endocrinol. 2016;8:298–304.
Frederiksen H, Kuiri-Hanninen T, Main KM, Dunkel L, Sankilampi U. A longitudinal study of urinary phthalate excretion in 58 full-term and 67 preterm infants from birth through 14 months. Environ Health Perspect. 2014;122:998–1005.
Strømmen K, Lyche JL, Blakstad EW, Moltu SJ, Veierød MB, Almaas AN, et al. Increased levels of phthalates in very low birth weight infants with septicemia and bronchopulmonary dysplasia. Environ Int. 2016;89-90:228–34.
Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res. 2011;69(5 Pt 2):11R–8R.
Montagna A, Nosarti C. Socio-emotional development following very preterm birth: pathways to psychopathology. Frontiers in Psychology. 2016;7:80. https://doi.org/10.3389/fpsyg.2016.00080.
Services DoHaH. Fourth national report on human exposure to environmental chemicals. Atlanta, GA; 2009.
Koch HM, Muller J, Angerer J. Determination of secondary, oxidised di-iso-nonylphthalate (DINP) metabolites in human urine representative for the exposure to commercial DINP plasticizers. J Chromatogr B Anal Technol Biomed Life Sci. 2007;847:114–25.
Madlinger-Lewis L, Reynolds L, Zarem C, Crapnell T, Inder T, Pineda R. The effects of alternative positioning on preterm infants in the neonatal intensive care unit: a randomized clinical trial. Res Dev Disabil. 2014;35:490–7.
Donauer S, Chen A, Xu Y, Calafat AM, Sjodin A, Yolton K. Prenatal exposure to polybrominated diphenyl ethers and polyfluoroalkyl chemicals and infant neurobehavior. J Pediatr. 2015;166:736–42.
Yolton K, Xu Y, Sucharew H, Succop P, Altaye M, Popelar A, et al. Impact of low-level gestational exposure to organophosphate pesticides on neurobehavior in early infancy: a prospective study. Environ Health. 2013;12:79.
Bernard L, Decaudin B, Lecoeur M, Richard D, Bourdeaux D, Cueff R, et al. Analytical methods for the determination of DEHP plasticizer alternatives present in medical devices: a review. Talanta. 2014;129:39–54.
Acknowledgements
A.S. is supported by a career development award, K23ES022268, from the National Institutes of Environmental Health Sciences (NIEHS) and a cooperative agreement, UG3OD023320, from the National Institutes of Health for the Environmental Influences on Child Health Outcomes (ECHO) program. Additional funding for the investigators and this project came through pilot grants from the Passport Foundation, the Mount Sinai Children’s Environmental Health Center, and an NIEHS center grant P30ES023515.
Funding
A.S. is supported by a career development award, K23ES022268, from the National Institutes of Environmental Health Sciences (NIEHS) and a cooperative agreement, UG3OD023320, from the National Institutes of Health for the Environmental Influences on Child Health Outcomes (ECHO) program. Additional funding for the investigators and this project came through pilot grants from the Passport Foundation, the Mount Sinai Children’s Environmental Health Center, and an NIEHS center grant P30ES023515.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Appendix 3
Directed acyclic graphs depicting covariate selection. Models based on WQS regressions involving NNNS subscales included covariates representing severity of illness at birth (base deficit on central blood sampling in the first 12 h of life), gestational age at birth, a variable representing composite medical illness in the NICU, and gender. For models based on DEHP exposure that did not take into accounte neurodevelopmental outcome (i.e., NNNS performance), gestational age at birth, status as a small for gestational age at birth, and infant gender were included as covariates in the model.
Rights and permissions
About this article
Cite this article
Stroustrup, A., Bragg, J.B., Busgang, S. et al. Sources of clinically significant neonatal intensive care unit phthalate exposure. J Expo Sci Environ Epidemiol 30, 137–148 (2020). https://doi.org/10.1038/s41370-018-0069-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-018-0069-2
Keywords
This article is cited by
-
Exposure of preterm neonates receiving total parenteral nutrition to phthalates and its impact on neurodevelopment at the age of 2 months
Scientific Reports (2023)
-
Environmental influences on child health outcomes: cohorts of individuals born very preterm
Pediatric Research (2023)
-
Commentary: …And a beer for the baby, please
Pediatric Research (2020)
-
Clinical validation of the Neonatal Infant Stressor Scale with preterm infant salivary cortisol
Pediatric Research (2020)
-
The changing spectrum of hypertension in premature infants
Journal of Perinatology (2019)