Abstract
Background/Objectives
Differences in gut microbiota, metabolites and immune markers have been observed between individuals with and without obesity. Our study determined the temporal association between infant fecal gut metabolites, sIgA and body mass index (BMI) z score of preschool children, independent of pre/postnatal factors.
Subjects/Methods
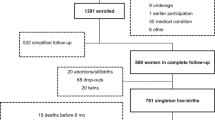
The study includes a subset of 647 infants from the CHILD Cohort Study (recruited between January 1, 2009, and December 31, 2012). Fecal metabolites and sIgA were measured at 3–4 months of age, and age and sex adjusted BMI z scores at 1 and 3 years of age. Associations between the metabolites, IgA, and child BMI z scores at age 1 and 3 years were tested using linear regression adjusted for pre/postnatal factors (breastfeeding, birthweight-for-gestational age, birthmode and IAP, solid food introduction).
Results
Mean BMI z score for all infants was 0.34 (SD 1.16) at 1 year (N = 647) and 0.71 (SD 1.06) at 3 years (N = 573). High fecal formate in infancy was associated with a significantly lower BMI z score (adjusted mean difference −0.23 (95% CI −0.42, −0.04)) and high butyrate was associated with a higher BMI z score (adjusted mean difference 0.21 (95% CI 0.01, 0.41)) at age 3 years only. The influence of formate and butyrate on BMI z score at age 3 were seen only in those that were not exclusively breastfed at stool sample collection (adjusted mean difference for high formate/EBF- group: −0.33 (95%CI −0.55, −0.10) and 0.25 (95% CI 0.02, 0.47) for high butyrate/EBF- group). No associations were seen between sIgA and BMI z score at age 1 or 3 years in adjusted regression models.
Conclusion and relevance
Differences in fecal metabolite levels in early infancy were associated with childhood BMI. This study identifies an important area of future research in understanding the pathogenesis of obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
A list of variables available in the CHILD Cohort Study is available at https://childstudy.ca/for-researchers/study-data/. Researchers interested in collaborating on a project and accessing CHILD Cohort Study data should contact the Study’s National Coordinating Center (NCC) to discuss their needs before initiating a formal request. To contact the NCC, please email child@mcmaster.ca. More information about data access for the CHILD Cohort Study can be found at https://childstudy.ca/forresearchers/data-access/.
References
Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 2016;315:2292–9.
Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91:1499s–505s.
Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. 2016;17:95–107.
Sommer F, Backhed F. The gut microbiota–masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–38.
Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012;7:91–109.
Karlsson CL, Onnerfalt J, Xu J, Molin G, Ahrne S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity. 2012;20:2257–61.
Bervoets L, Van Hoorenbeeck K, Kortleven I, Van Noten C, Hens N, Vael C, et al. Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathog. 2013;5:10.
Koleva PT, Bridgman SL, Kozyrskyj AL. The infant gut microbiome: evidence for obesity risk and dietary intervention. Nutrients. 2015;7:2237–60.
Payne AN, Chassard C, Zimmermann M, Muller P, Stinca S, Lacroix C. The metabolic activity of gut microbiota in obese children is increased compared with normal-weight children and exhibits more exhaustive substrate utilization. Nutr Diabetes. 2011;1:e12.
Kumari M, Kozyrskyj AL. Gut microbial metabolism defines host metabolism: an emerging perspective in obesity and allergic inflammation. Obes Rev. 2017;18:18–31.
Murugesan S, Nirmalkar K, Hoyo-Vadillo C, Garcia-Espitia M, Ramirez-Sanchez D, Garcia-Mena J. Gut microbiome production of short-chain fatty acids and obesity in children. Eur J Clin Microbiol Infect Dis. 2018;37:621–5.
Gutzeit C, Magri G, Cerutti A. Intestinal IgA production and its role in host-microbe interaction. Immunol Rev. 2014;260:76–85.
Luck H, Khan S, Kim JH, Copeland JK, Revelo XS, Tsai S, et al. Gut-associated IgA(+) immune cells regulate obesity-related insulin resistance. Nat Commun. 2019;10:3650.
Subbarao P, Anand SS, Becker AB, Befus AD, Brauer M, Brook JR, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) Study: examining developmental origins of allergy and asthma. Thorax. 2015;70:998–1000.
Tun HM, Konya T, Takaro TK, Brook JR, Chari R, Field CJ, et al. Exposure to household furry pets influences the gut microbiota of infant at 3-4 months following various birth scenarios. Microbiome. 2017;5:40.
Emwas AH. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol Biol. 2015;1277:161–93.
Forrest BD. Effects of sample processing on the measurement of specific intestinal IgA immune responses. Vaccine. 1992;10:802–5.
WHO. Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85.
Kim KN, Yao Y, Ju SY. Short chain fatty acids and fecal microbiota abundance in humans with obesity: a systematic review and meta-analysis. Nutrients. 2019;11:2512.
Kang LJ, Koleva PT, Field CJ, Giesbrecht GF, Wine E, Becker AB, et al. Maternal depressive symptoms linked to reduced fecal Immunoglobulin A concentrations in infants. Brain Behav Immun. 2018;68:123–31.
Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67:850–7.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B. 1995;57:289–300.
Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, et al. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ Microbiol. 2017;19:95–105.
Murugesan S, Ulloa-Martinez M, Martinez-Rojano H, Galvan-Rodriguez FM, Miranda-Brito C, Romano MC, et al. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur J Clin Microbiol Infect Dis. 2015;34:1337–46.
Baker JL, Olsen LW, Sørensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–37.
Meyer JF, Larsen SB, Blond K, Damsgaard CT, Bjerregaard LG, Baker JL. Associations between body mass index and height during childhood and adolescence and the risk of coronary heart disease in adulthood: a systematic review and meta-analysis. Obes Rev. 2021;22:e13276.
Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–84.
Zhang L, Liu C, Jiang Q, Yin Y. Butyrate in energy metabolism: there is still more to learn. Trends Endocrinol Metab. 2021;32:159–69.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
de la Cuesta-Zuluaga J, Mueller NT, Álvarez-Quintero R, Velásquez-Mejía EP, Sierra JA, Corrales-Agudelo V, et al. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients. 2018;11:51.
Ferrer-Picón E, Dotti I, Corraliza AM, Mayorgas A, Esteller M, Perales JC, et al. Intestinal inflammation modulates the epithelial response to butyrate in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2020;26:43–55.
Pietzke M, Meiser J, Vazquez A. Formate metabolism in health and disease. Mol Metab. 2020;33:23–37.
Badillo-Suárez PA, Rodríguez-Cruz M, Nieves-Morales X. Impact of metabolic hormones secreted in human breast milk on nutritional programming in childhood obesity. J Mammary Gland Biol Neoplasia. 2017;22:171–91.
Savino F, Liguori SA, Fissore MF, Oggero R. Breast milk hormones and their protective effect on obesity. Int J Pediatr Endocrinol. 2009;2009:327505.
Pallaro A, Barbeito S, Taberner P, Marino P, Franchello A, Strasnoy I, et al. Total salivary IgA, serum C3c and IgA in obese school children. J Nutr Biochem. 2002;13:539.
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–45.
Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8.
Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19:29–41.
Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, et al. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000;66:1654–61.
Forbes JD, Azad MB, Vehling L, Tun HM, Konya TB, Guttman DS, et al. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. 2018;172:e181161.
Xu Y, Wang N, Tan HY, Li S, Zhang C, Feng Y. Function of Akkermansia muciniphila in obesity: interactions with lipid metabolism, immune response and gut systems. Front Microbiol. 2020;11:219.
Tsukuda N, Yahagi K, Hara T, Watanabe Y, Matsumoto H, Mori H, et al. Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. Isme j. 2021;15:2574–90.
Korpela K, Zijlmans MA, Kuitunen M, Kukkonen K, Savilahti E, Salonen A, et al. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome. 2017;5:26.
Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8.
Belzer C, Chia LW, Aalvink S, Chamlagain B, Piironen V, Knol J, et al. Microbial metabolic networks at the mucus layer lead to diet- independent butyrate and vitamin B(12) production by intestinal symbionts. mBio. 2017;8:e00770–17.
Karav S, Casaburi G, Frese SA. Reduced colonic mucin degradation in breastfed infants colonized by Bifidobacterium longum subsp. infantis EVC001. FEBS Open Bio. 2018;8:1649–57.
Acknowledgements
We thank the CHILD Cohort Study (CHILD) participant families for their dedication and commitment to advancing health research as well as the whole CHILD study team, which includes interviewers, nurses, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, and receptionists. We also thank Susan Goruk for her assistance in sIgA analysis, Theodore Konya for his work on the microbiota sample processing and analysis and Jennifer Petrie for her assistance in data entry. The Canadian Institutes of Health Research (CIHR) and the Allergy, Genes and Environment Network of Centers of Excellence provided core funding for the Canadian Healthy Infant Longitudinal Development (CHILD) Study. This research was specifically funded by CIHR Microbiome Initiative team grant 227312 and the Women and Children’s Health Research Institute at the University of Alberta.
Author information
Authors and Affiliations
Contributions
ALK, SLB, CJF, AMH, PJM, ES, PS, SET, JAS were involved in the study concept and design. CJF, RM, PJM, TJM, ES, SET, JAS and DSW were involved in the acquisition of data. SLB conducted the data cleaning and statistical analysis and SLB and ALK drafted of the manuscript. All authors provided critical revision of the manuscript for important intellectual content and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bridgman, S.L., Malmuthuge, N., Mandal, R. et al. Childhood body mass index and associations with infant gut metabolites and secretory IgA: findings from a prospective cohort study. Int J Obes 46, 1712–1719 (2022). https://doi.org/10.1038/s41366-022-01183-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01183-3
This article is cited by
-
Delayed gut microbiota maturation in the first year of life is a hallmark of pediatric allergic disease
Nature Communications (2023)