Abstract
Background
For the same BMI, South Asians have a higher body fat percentage, a higher liver fat content and a more adverse metabolic profile than whites. South Asians may have a lower fat oxidation than whites, which could result in an unfavorable metabolic profile when exposed to increased high-fat foods consumption and decreased physical activity as in current modern lifestyle.
Objective
To determine substrate partitioning, liver fat accumulation and metabolic profile in South Asian and white men in response to overfeeding with high-fat diet under sedentary conditions in a respiration chamber.
Design
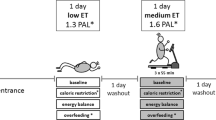
Ten South Asian men (BMI, 18–29 kg/m2) and 10 white men (BMI, 22–33 kg/m2), matched for body fat percentage, aged 20–40 year were included. A weight maintenance diet (30% fat, 55% carbohydrate, and 15% protein) was given for 3 days. Thereafter, a baseline measurement of liver fat content (1H-MRS) and blood parameters was performed. Subsequently, subjects were overfed (150% energy requirement) with a high-fat diet (60% fat, 25% carbohydrate, and 15% protein) over 3 consecutive days while staying in a respiration chamber mimicking a sedentary lifestyle. Energy expenditure and substrate use were measured for 3 × 24-h. Liver fat and blood parameters were measured again after the subjects left the chamber.
Results
The 24-h fat oxidation as a percentage of total energy expenditure did not differ between ethnicities (P = 0.30). Overfeeding increased liver fat content (P = 0.02), but the increase did not differ between ethnicities (P = 0.64). In South Asians, overfeeding tended to increase LDL-cholesterol (P = 0.08), tended to decrease glucose clearance (P = 0.06) and tended to elevate insulin response (P = 0.07) slightly more than whites.
Conclusions
Despite a similar substrate partitioning and similar accretion of liver fat, overfeeding with high-fat under sedentary conditions tended to have more adverse effects on the lipid profile and insulin sensitivity in South Asians.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32:1431–7.
Chopra M, Galbraith S, Darnton-Hill I. A global response to a global problem: the epidemic of overnutrition. Bull World Health Organ. 2002;80:952–8.
Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–8.
Forouhi NG, Jenkinson G, Thomas EL, Mullick S, Mierisova S, Bhonsle U, et al. Relation of triglyceride stores in skeletal muscle cells to central obesity and insulin sensitivity in European and South Asian men. Diabetologia. 1999;42:932–5.
Chandalia M, Lin P, Seenivasan T, Livingston EH, Snell PG, Grundy SM, et al. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS ONE. 2007;2:e812.
Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, et al. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes. 2008;57:1166–75.
Rush EC, Freitas I, Plank LD. Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutr. 2009;102:632–41.
Wulan SN, Westerterp KR, Plasqui G. Dietary and 24-h fat oxidation in Asians and whites who differ in body composition. Am J Clin Nutr. 2012;95:1335–41.
Andreasen CH, Stender-Petersen KL, Mogensen MS, Torekov SS, Wegner L, Andersen G, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57:95–101.
King GA, Fitzhugh EC, Bassett DR Jr., McLaughlin JE, Strath SJ, Swartz AM, et al. Relationship of leisure-time physical activity and occupational activity to the prevalence of obesity. Int J Obes Relat Metab Disord. 2001;25:606–12.
Kim IY, Park S, Trombold JR, Coyle EF. Effects of moderate- and intermittent low-intensity exercise on postprandial lipemia. Med Sci Sports Exerc. 2014;46:1882–90.
Bergouignan A, Kealey EH, Schmidt SL, Jackman MR, Bessesen DH. Twenty-four hour total and dietary fat oxidation in lean, obese and reduced-obese adults with and without a bout of exercise. PLoS ONE. 2014;9:e94181.
Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Role of glycogen-lowering exercise in the change of fat oxidation in response to a high-fat diet. Am J Physiol. 1997;273:E623–629.
Lefai E, Blanc S, Momken I, Antoun E, Chery I, Zahariev A, et al. Exercise training improves fat metabolism independent of total energy expenditure in sedentary overweight men, but does not restore lean metabolic phenotype. Int J Obes. 2017;41:1728–36.
Westerbacka J, Lammi K, Hakkinen AM, Rissanen A, Salminen I, Aro A, et al. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab. 2005;90:2804–9.
van Herpen NA, Schrauwen-Hinderling VB, Schaart G, Mensink RP, Schrauwen P. Three weeks on a high-fat diet increases intrahepatic lipid accumulation and decreases metabolic flexibility in healthy overweight men. J Clin Endocrinol Metab. 2011;96:E691–695.
Wulan SN, Westerterp KR, Plasqui G. Metabolic profile before and after short-term overfeeding with a high-fat diet: a comparison between South Asian and White men. Br J Nutr. 2014;111:1853–61.
Westerterp KR, Wouters L, van Marken Lichtenbelt WD. The Maastricht protocol for the measurement of body composition and energy expenditure with labeled water. Obes Res. 1995;3(Suppl 1):49–57.
Siri WE. The gross composition of the body. Adv Biol Med Phys. 1956;4:239–80.
Bonomi AG, Plasqui G, Goris AH, Westerterp KR. Estimation of free-living energy expenditure using a novel activity monitor designed to minimize obtrusiveness. Obesity. 2010;18:1845–51.
Joosen AM, Bakker AH, Zorenc AH, Kersten S, Schrauwen P, Westerterp KR. PPARgamma activity in subcutaneous abdominal fat tissue and fat mass gain during short-term overfeeding. Int J Obes. 2006;30:302–7.
Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Moonen-Kornips E, Schaart G, Mustard KJ, et al. Intramyocellular lipid content and molecular adaptations in response to a 1-week high-fat diet. Obes Res. 2005;13:2088–94.
Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet. Am J Clin Nutr. 1997;66:276–82.
Schoffelen PF, Westerterp KR, Saris WH, Ten Hoor F. A dual-respiration chamber system with automated calibration. J Appl Physiol. 1997;83:2064–72.
Brouwer E. On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (oxygen intake and carbonic acid output) and urine-N. Acta Physiol Pharmacol Neerl. 1957;6:795–802.
Hochstenbach-Waelen A, Veldhorst MA, Nieuwenhuizen AG, Westerterp-Plantenga MS, Westerterp KR. Comparison of 2 diets with either 25 or 10% of energy as casein on energy expenditure, substrate balance, and appetite profile. Am J Clin Nutr. 2009;89:831–8.
Wouters-Adriaens MP, Westerterp KR. Low resting energy expenditure in Asians can be attributed to body composition. Obesity. 2008;16:2212–6.
Westerterp KR, Plasqui G. Physical activity and human energy expenditure. Curr Opin Clin Nutr Metab Care. 2004;7:607–13.
van Werven JR, Hoogduin JM, Nederveen AJ, van Vliet AA, Wajs E, Vandenberk P, et al. Reproducibility of 3.0 Tesla magnetic resonance spectroscopy for measuring hepatic fat content. J Magn Reson Imaging. 2009;30:444–8.
Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–22.
Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43.
Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, et al. JAVA-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–52.
Hamilton G, Yokoo T, Bydder M, Cruite I, Schroeder ME, Sirlin CB, et al. In vivo characterization of the liver fat (1)H MR spectrum. NMR Biomed. 2011;24:784–90.
Sievenpiper JL, Jenkins DJ, Josse RG, Vuksan V. Dilution of the 75-g oral glucose tolerance test improves overall tolerability but not reproducibility in subjects with different body compositions. Diabetes Res Clin Pract. 2001;51:87–95.
Report of The Expert Committee on Diagnostic and Classification of Diabetes Mellitus. Diabetes Care. 1997,20:1183–97.
American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl 1):S11–61.
Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2.
Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24:539–48.
Jans A, Sparks LM, van Hees AM, Gjelstad IM, Tierney AC, Riserus U, et al. Transcriptional metabolic inflexibility in skeletal muscle among individuals with increasing insulin resistance. Obesity. 2011;19:2158–66.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Bergouignan A, Momken I, Lefai E, Antoun E, Schoeller DA, Platat C, et al. Activity energy expenditure is a major determinant of dietary fat oxidation and trafficking, but the deleterious effect of detraining is more marked than the beneficial effect of training at current recommendations. Am J Clin Nutr. 2013;98:648–58.
Flatt JP. Use and storage of carbohydrate and fat. Am J Clin Nutr. 1995;61:952S–959S.
Flatt JP. Glycogen levels and obesity. Int J Obes Relat Metab Disord. 1996;20(Suppl 2):S1–11.
Westerterp KR. Metabolic adaptations to over–and underfeeding–still a matter of debate? Eur J Clin Nutr. 2013;67:443–5.
Flatt JP. Carbohydrate-fat interactions and obesity examined by a two-compartment computer model. Obes Res. 2004;12:2013–22.
van der Meer RW, Hammer S, Lamb HJ, Frolich M, Diamant M, Rijzewijk LJ, et al. Effects of short-term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. J Clin Endocrinol Metab. 2008;93:2702–8.
Heath RB, Karpe F, Milne RW, Burdge GC, Wootton SA, Frayn KN. Selective partitioning of dietary fatty acids into the VLDL TG pool in the early postprandial period. J Lipid Res. 2003;44:2065–72.
Lindeboom L, Nabuurs CI, Hesselink MK, Wildberger JE, Schrauwen P, Schrauwen-Hinderling VB. Proton magnetic resonance spectroscopy reveals increased hepatic lipid content after a single high-fat meal with no additional modulation by added protein. Am J Clin Nutr. 2015;101:65–71.
Anand SS, Tarnopolsky MA, Rashid S, Schulze KM, Desai D, Mente A, et al. Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: the Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE). PLoS ONE. 2011;6:e22112.
Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–6.
Sniderman AD, De Graaf J, Couture P, Williams K, Kiss RS, Watts GF. Regulation of plasma LDL: the apoB paradigm. Clin Sci. 2009;118:333–9.
Brons C, Jensen CB, Storgaard H, Hiscock NJ, White A, Appel JS, et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol. 2009;587:2387–97.
Sharman MJ, Gomez AL, Kraemer WJ, Volek JS. Very low-carbohydrate and low-fat diets affect fasting lipids and postprandial lipemia differently in overweight men. J Nutr. 2004;134:880–5.
Hu T, Mills KT, Yao L, Demanelis K, Eloustaz M, Yancy WS Jr., et al. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2012;176(Suppl 7):S44–54.
Merchant AT, Anand SS, Kelemen LE, Vuksan V, Jacobs R, Davis B, et al. Carbohydrate intake and HDL in a multiethnic population. Am J Clin Nutr. 2007;85:225–30.
Grundy SM, Abate N, Chandalia M. Diet composition and the metabolic syndrome: what is the optimal fat intake? Am J Med. 2002;113(Suppl 9B):25S–29S.
Grundy SM. Hypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndrome. Am J Cardiol. 1998;81:18B–25B.
Bakker LE, van Schinkel LD, Guigas B, Streefland TC, Jonker JT, van Klinken JB, et al. A 5-day high-fat, high-calorie diet impairs insulin sensitivity in healthy, young South Asian men but not in Caucasian men. Diabetes. 2014;63:248–58.
Acknowledgements
We gratefully thank Paul Schoffelen, Loek Wouters, Wendy Sluijsmans, Hasibe Aydeniz, and the late Jos Stegen for technical assistance and analysis. We thank Henk Schoenmakers, Roland Kersemakers, and the technicians of the MRI Unit, Academic Hospital Maastricht for technical assistance. We deeply appreciate and thank all subjects who participated in the study. SNW was supported by a fellowship from The Directorate General of Higher Education, The Ministry of Research Technology and Higher Education of The Republic of Indonesia. VBS-H was supported by a veni grant (91611136) for innovative research from the Netherlands Organization for Scientific Research. The study was approved by The Medical Ethics Committee of Maastricht University, MEC No. 10-3-013 and registered in the public trial registry www.ccmo.nl No. NL31217.068.10.
Author information
Authors and Affiliations
Contributions
SNW: conducted the research, performed the data analysis, and wrote the paper; KRW, VBS-H, and GP: designed the study, interpreted the data, and reviewed the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wulan, S.N., Schrauwen-Hinderling, V.B., Westerterp, K.R. et al. Substrate utilization and metabolic profile in response to overfeeding with a high-fat diet in South Asian and white men: a sedentary lifestyle study. Int J Obes 44, 136–146 (2020). https://doi.org/10.1038/s41366-019-0368-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0368-2
This article is cited by
-
Determinants of the postprandial triglyceride response to a high-fat meal in healthy overweight and obese adults
Lipids in Health and Disease (2021)
-
Effect of Over- and Underfeeding on Body Composition and Related Metabolic Functions in Humans
Current Diabetes Reports (2019)