Abstract
Background:
Prior conclusions that autologous neonatal red blood cells (RBC) have substantially shorter lifespans than allogeneic adult RBCs were not based on direct comparison of autologous neonatal vs. allogeneic adult RBCs performed concurrently in the same infant. Biotin labeling of autologous neonatal RBCs and allogeneic adult donor RBCs permits concurrent direct comparison of autologous vs. allogeneic RBC lifespan.
Methods:
RBCs from 15 allogeneic adult donors and from 15 very-low-birth-weight (VLBW) neonates were labeled at separate biotin densities and transfused simultaneously into the 15 neonates. Two mathematical models that account for the RBC differences were employed to estimate lifespans for the two RBC populations.
Results:
Mean ± SD lifespan for adult allogeneic RBC was 70.1 ± 19.1 d, which is substantially shorter than the 120 d lifespan of both autologous and adult allogeneic RBC in healthy adults. Mean ± SD lifespan for neonatal RBC was 54.2 ± 11.3 d, which is only about 30% shorter than that of the adult allogeneic RBCs.
Conclusion:
This study provides evidence that extrinsic environmental factors primarily determine RBC survival (e.g., small bore of the capillaries of neonates, rate of oxygenation/deoxygenation cycles) rather than factors intrinsic to RBC.
Similar content being viewed by others
Main
Red blood cells (RBC) from neonates differ substantially from those in healthy adults. Neonatal RBCs are produced during a period of stimulated erythropoiesis that is needed to rapidly expand blood volume in response to rapid somatic growth (1). In contrast, in healthy adults, RBCs are produced under steady state (SS) erythropoiesis associated with stable body weight and blood volume. Perhaps in part because of these differences in erythropoietic rates, neonatal RBCs are less deformable, larger in size, and more fragile than adult RBCs (2,3). These intrinsic RBC differences could affect RBC lifespan.
The environment in which the RBCs circulate might also affect RBC lifespan. The mean in vivo lifespan (a term used interchangeably here with long-term survival) of adult autologous and allogeneic RBCs in healthy adults is approximately 120 d (4,5). In contrast to the interpretation of previous neonatal studies, our recent studies indicate that adult RBCs transfused into an anemic infant survive much less than 120 d (6). Further, if neonatal RBCs are transfused into healthy adults, these RBCs survive for longer than they would have in the infant donor (7), suggesting that environmental circulatory conditions may play a major and unappreciated role in relative RBC survival.
The most commonly used RBC population labels are the radionuclides 51Cr and 32P. These labels allow only one population of RBCs to be studied at a time in a given subject, thus precluding simultaneous direct head-to-head comparison of two populations (e.g., neonatal vs. adult RBC). Specifically, after an anemic infant is transfused with adult donor RBCs, there are two separate RBC populations present in circulation. To concurrently compare lifespan of the two RBC populations, an alternative method capable of separately identifying and quantifying persistence in circulation of each of the two RBC populations is needed. Biotin is a nonradioactive, nontoxic vitamin that has been used to covalently label proteins on the outer surface of the RBC membrane in RBC survival studies (5). The biotin labeling method is well suited to study multiple (up to 4) RBC populations simultaneously in the same subject with separate populations of RBCs being labeled at discrete biotin density levels (8,9,10).
The objectives of the present study were the following: (i) to develop a quantitative mathematical model to accurately estimate concurrent lifespans of neonatal and adult RBCs that have been transfused in newborn infants and (ii) to apply this method to determine the lifespan of neonatal and adult RBCs in very-low-birth-weight (VLBW) neonates when tracked concurrently. Such a method can account for confounding factors that include phlebotomy blood loss, RBC transfusion, and RBC volume expansion resulting from somatic growth.
Methods
Subjects
The BioRBC tracking data from 15 VLBW preterm anemic infants previously reported (6) were used in this study. All infants were born at <29 wk gestation age (GA) and were cared for in the Neonatal Intensive Care Unit (NICU) at the University of Iowa (UI) Children’s Hospital. The University of Iowa Human Subject Internal Review Board approved the study. Inclusion criteria included treatment with expectation of survival and the presence of respiratory distress severe enough to require mechanical ventilation. Exclusion criteria included clinical evidence of diffuse intravascular coagulation, thrombosis, hematological disease other than anemia attributable to either phlebotomy loss, prematurity, or both, and an emergent transfusion requirement that did not allow controlled sampling. For each neonate, at least one parent or legal guardian provided written informed consent as a part of the ongoing consent process.
Biotinylation of RBCs and Their Flow Cytometric Analysis
Neonatal and adult RBCs were labeled at two discrete biotin density levels as previously described ( Figure 1 ) (8,9). To avoid potential bias, the biotin density levels were randomized between the adult allogeneic and the neonatal autologous using a balanced design. The percent of BioRBCs in post-transfusion blood samples was determined by flow cytometric enumeration after staining with Avidin conjugated with Alexa Fluor 488 (8,9).
Study protocol diagram of RBC biotinylation and population enumeration by flow cytometry. Allogeneic adult donor RBCs and autologous neonatal RBCs were labeled at discreet biotin density levels and and transfused to VLBW anemic study subjects at the end of the first (unlabeled) RBC transfusion given to treat anemia. Post-transfusion blood samples leftover from laboratory testing were analyzed by flow cytometry to determine the fraction of biotin-labeled adult and neonatal RBCs that remained in circulation.
Study Protocol
All RBC transfusions were ordered by the subject’s attending neonatologist in accordance with UI NICU clinical guidelines (1). In this study, RBCs from two sources were labeled with biotin: (i) the first allogeneic adult RBC transfusion and (ii) autologous neonatal blood. These BioRBCs were sequentially transfused to the study subjects after the clinical transfusion as previously described (11). From birth to the end of the BioRBC study period, blood samples left over from the clinically indicated laboratory studies were weighed and recorded immediately after collection. The weight of the blood collection tube was subtracted from the total weight of tube and blood sample, and this blood sample weight was converted to the volume of blood removed using an estimated specific gravity of 1.05 for whole blood (11). The mass of Hb removed with each phlebotomy was calculated by multiplying the volume of blood removed times the Hb concentration in that blood sample. Subjects who became anemic again after the initial transfusion of unlabeled RBC and BioRBC received additional unlabeled RBC transfusions at later times when clinically indicated. For all transfusions, the volume of packed RBCs (85% Hct) administered was 15 ml/kg; the time of RBC transfusions were recorded for use in the model analysis.
The Model
Model for adult donor RBC survival. RBCs from healthy adult donors produced under normal hematologic steady state conditions were isolated, labeled, and transfused into the study infants. The model takes into account that a fraction of the original pool of the initially transfused labeled RBCs will be removed from the circulation each day, leading to a linear survival curve similar to that observed in transfused adults in steady state erythropoiesis (5). This linear survival curve can then be extrapolated to the time axis to estimate the adult donor RBC lifespan. The model to describe this linear decline of labeled RBCs/Hb after transfusion at time t is given as:
(1)

where HbL(t) represents the amount of Hb present in labeled RBCs at time t and, L(0) is the lifespan of the labeled adult donor RBCs.
Model for neonatal RBC survival. In contrast to adult RBCs, neonatal RBCs are produced under non-steady state (non-SS) conditions. To accurately model the survival of neonatal RBCs, a model that accounts for non-SS RBC production is required. Such a model has been derived by us previously (12) and was employed in this study. In brief, in utero growth was estimated using the birth weight as a function of GA (13) and can be expressed as follows:
(2)

where GA is the gestational age at birth measured in days and A, B, C, D, E, M and γ are fixed parameters that were set to values previously validated for VLBW infants ( Table 1 ) (12). The erythropoiesis rate R(t) is assumed to be proportional to the body weight. Accordingly, R(t) can be expressed as:
(3)

where BW(t) is the body weight at time t and k is a scaling factor that relates the in utero growth to fetal erythropoiesis rate. The final model to calculate the amount of Hb present in neonatal labeled RBCs remaining in circulation at any time t can then be expressed as:
(4)

where p=154 d (Equation 2) and,
(5)


(7)

(8)

where MCH is the mean corpuscular Hb for neonatal RBCs; MCH was set equal to 37.5 pg/cell, a value previously determined by this group (14). In both models, the disposition of RBCs (and the Hb in those RBCs) was assumed to be lifespan based; specifically, we assumed that RBCs were removed from circulation through cellular aging/senescence (4,15,16,17).
Accurately Accounting for Phlebotomies in the Models
All critically ill newborn infants experience substantial phlebotomy losses as a result of clinical testing; accordingly, Equations 1 and 4 must be corrected to accurately account for the loss of BioRBCs due to phlebotomies. Loss of labeled RBCs from circulation was accounted for as previously described (14,18,19). Consider that the ith phlebotomy was performed at time tpi and that a certain fraction of Hb was removed from circulation. After the ith phlebotomy, the fraction remaining FRMi.is given by Equation 9:
(9)

where HbRMi is the hemoglobin removed due to the ith phlebotomy at time tpi. For multiple phlebotomies, the phlebotomy correction factor can be calculated as shown in Equation 10,
(10)

where the fraction remaining after each phlebotomy are ordered from the first to the last phlebotomy, j is the first phlebotomy after entry of the RBCs into the systemic circulation and q is the last phlebotomy prior to the current time t. In this case, j represents the first phlebotomy after the BioRBCs are introduced into the circulation, and thus the Equation 10 now can be written as Equation 11.
(11)

Data Analysis
Data analyses were performed in R version 3.0.3 using the RStudio integrated development environment (20,21). All modeling were conducted using WINFUNFIT, a Windows (Microsoft) version evolved from the general nonlinear regression program FUNFIT (22), using ordinary least squares fit to Hb amount vs. time profile for each subject. To characterize the uncertainty in the estimates of the individual subject parameters, the standard deviation (SD) of the estimates were calculated for each parameter. Neonatal and adult RBC lifespans were compared using a two tailed, paired t-test. Statistical differences were considered to be significant for values of P<0.05.
Results
Subject Characteristics
Five males (all singletons) and 10 females (7 singletons and 3 twins) were studied. At the time of the BioRBC transfusion, the mean body weight of the 15 anemic VLBW infants was 0.742 kg (range 0.494–1.042 kg), and the mean GA was 25 wk (range 23–27). During the BioRBC study period, subjects received a mean of five additional RBC transfusions (range 1–9). The mean hematocrit at the time of the BioRBC transfusion was 32.7% (range 28.6–39.8%).
Model Fit to BioRBC Data
The non-SS neonatal RBC survival model (Equation 4) fit to the Hb disappearance curves for two representative subjects are displayed in Figure 2a , b . General agreement between the model fit and the infant Hb amount data was observed. The mean (± SD) model estimated RBC lifespan of neonatal RBCs in neonatal infants was 54.2 ± 11.3 d. The mean estimated value of k, the scaling parameter (Equation 3), was 0.92 × 107 RBCs/d/g.
Model fit to Hb amount vs. time data. Panels a and b depict the fit (solid line) of the non-SS neonatal RBC survival model (Equation 4) to the empirical Hb amount circulating in autologous neonatal BioRBCs (open squares) for two representative study subjects. Panels c and d depict the fit (solid line) of the steady-state adult RBC survival model (Equation 1) to the empirical Hb in circulating allogeneic adult donor BioRBCs (open squares) for the same two subjects. Agreement is good between the model fits and the Hb amounts in circulation for each population of RBC (adult and infant) for both subjects.
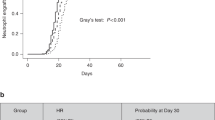
The steady state adult RBC survival model (Equation 1) fit to the adult Hb disappearance curves for the same two representative subjects are displayed in Figure 2c , d . The mean model estimated RBC lifespan of adult donor RBCs in neonatal infants was 70.1 ± 19.1 d. Although the mean lifespans of both adult RBCs and neonatal RBCs in neonates were substantially shorter than that in healthy adults (~120 d), the survival of adult RBCs was still significantly greater than that of neonatal RBCs (P < 0.05, Figure 3 ).
To assess the potential contribution of developmental factors to determining RBC lifespan, the relationship of infant body weight (as a marker of vascular volume as well as maturation) to RBC lifespan was assessed by plotting mean estimated lifespans for adult RBCs were against body weight for the 15 infants ( Figure 4 ). A significant positive correlation was observed between in vivo allogeneic adult RBC lifespan and infant body weight (P < 0.05), but not between infant RBC lifespan and infant body weight (data not shown).
Discussion
In this study, autologous infant and allogeneic adult RBCs labeled at discrete biotin density levels were transfused to anemic VLBW preterm infants to directly and concurrently determine the RBC in vivo lifespan. In these neonates, RBC lifespans of both adult and neonatal RBCs were much shorter than the 120 d reported for adult RBC (autologous and allogeneic) in healthy adults. These observations provide strong evidence that some aspect (or aspects) in the infant circulatory environment substantially shortens the survival of adult RBCs. In addition, lifespan of the adult RBCs was modestly longer (about 30%) than that of the neonatal RBCs providing evidence that some difference in an intrinsic characteristic of the two populations of RBCs is a significant, but less important determinant of lifespan.
Neonatal RBCs and adult RBCs are produced under different conditions (i.e., SS where the erythropoiesis rate is constant vs. non-SS where the RBC production rate varies with time); thus, simple comparison of the two BioRBC disappearance curves will be misleading concerning true RBC lifespan. To accurately account for this complexity, two separate models were employed in the present study for estimating the lifespans of the two RBC populations: one describing the elimination of infant RBCs produced under non-SS conditions and the other describing the elimination of adult RBCs produced under SS conditions. Additional sophistication of the models was required to account for the complexity of the clinical circumstances of these subjects. The factors that contribute to this complexity include additional clinical transfusions administered to the infants during the lifespans of the BioRBCs, multiple phlebotomies, and blood volume increasing in response to somatic growth. All of these can perturb the BioRBC survival curves. In this study, all of these confounders were accounted for in our model estimation of the lifespans of the two RBC populations.
Our results and their interpretations are consistent with observations of RBC survival in other clinical situations. Indeed, a substantial effect of the circulatory environment on RBC survival has been previously reported based on RBC survival in cross-transfusion studies. When healthy recipients were transfused with RBCs from uremic patients, the uremic RBC survival increased (relative to autologous survival in uremic patients), whereas survival of RBCs from the healthy donors decreased in uremic patients (relative to autologous survival in the healthy donors) (23,24). Similarly, fetal RBCs from a fetomaternal hemorrhage had a lifespan in the maternal circulation close to that of adult RBCs (25). These studies indicate that the environment in which the RBCs circulate is the major determinant of long-term survival of RBCs.
We postulate that the shortened RBC lifespan of allogeneic adult RBCs in anemic VLBW infant circulation is attributable to a substantial environmental effect. Although the exact mechanism or mechanisms causing the shortening remains unclear, we speculate that one mechanism could be the increased mechanical shear damage of adult RBCs in infant circulation. A previous study in which adult donor RBCs were transfused to severely anemic D+ fetuses found that adult RBCs had decreased deformability after being introduced into the fetal environment (26). The impaired deformability would shorten the adult RBC survival due to the increased mechanical stress during the passage through the capillaries. A significant increase of the cholesterol-to-phospholipid (C/P) ratio in adult RBCs was considered as the primary cause for the decreased deformability of the donor RBCs. In addition, donor RBCs also undergo a larger number of capillary passages per unit time in the infant circulation compared to that in healthy adults. The combination of decreased RBC deformability and increased number of capillary passages of adult RBCs in infant circulation would cause a cumulative mechanical shear damage that could lead to shortened survival of adult RBCs in VLBW infant circulation.
The results from this study provide strong evidence for a substantial effect of the anemic premature infant’s circulation in decreasing RBC lifespan. Because allogeneic adult RBCs have substantially reduced lifespan, the number of allogeneic donor RBCs transfusions that anemic infants receive is greater relative to anemic adults (assuming that other factors such as laboratory phlebotomy loss were the same). In light of our observation that the lifespan of allogeneic adult RBCs is only about 30% longer in infants than autologous neonatal RBCs, we contend that clinical strategies such as delayed cord clamping and umbilical cord milking are almost as effective (per gram of Hb) as allogeneic donor RBC transfusions for VLBW preterm infants.
Because the mean adult RBC lifespan in infant circulation significantly increased with infant body weight ( Figure 4 ), we deduce that allogeneic donor RBCs will survive longer in larger infants as compared to smaller infants. We hypothesize that this reduced survival of allogeneic donor RBCs in smaller infants contributes to the greater number of RBC transfusions required for these VLBW and extremely low birth weight (< 1,000 g) infants (27,28,29).
Our study has several limitations. The total RBC count could not be measured at the time of each BioRBC sample because of sample volume and economic limitations. Instead, the Hb present in the BioRBCs was calculated from the measured BioRBC enrichment data, using the measured blood volume and infant body weight data. The estimated Hb present in the BioRBCs was subsequently modeled instead of the number of BioRBCs present in the infant circulation. This study assumed that the erythropoiesis rate R(t) to be proportional to the body weight. In contrast to healthy adults, neonatal RBCs are produced during a period of stimulated erythropoiesis that is needed to rapidly expand blood volume in response to rapid growth. This rapid growth increases the demand for oxygenation of the tissues and must be met by a proportional increase in rate of erythropoiesis. Further experimental evidence including studies with fetal sampling is needed to validate this assumption. Another limitation of the present study is that the sample size of 15 study subjects is relatively small; yet, considering the difficulty of studying these infants, 15 is a substantial number, and the observations offered are novel. Based on demographics and clinical characteristics, these VLBW study subjects appear to be representative of most VLBW preterm infants. In future studies, a larger number of study subjects encompassing a greater GA and body weight spectrum would improve confidence in the generalizability of the interpretations and postulated mechanisms.
In summary, the present study introduces a quantitative method to estimate in vivo RBC lifespan of neonatal RBCs produced under non-SS conditions and of adult RBCs produced under SS conditions as well as accounting for the major confounding factors including multiple phlebotomies, clinical transfusions, and RBC volume increase in response to somatic growth. The method was successfully applied to estimate the in vivo RBC lifespan of allogeneic adult RBCs and autologous infant RBCs in anemic VLBW preterm infants.
Author Contributions
D.N., R.L.S., and J.A.W. performed experiments; D.J.K., R.L.S., D.N., and J.A.W. analyzed data. D.J.K. prepared the first draft of the manuscript. P.V-P, D.N., R.L.S., J.A.W., and D.M.M. provided additional ideas, discussions, and helped in critical review. P.V-P, J.A.W., and D.M.M. obtained funding for this work. All authors approved the final version of the manuscript.
Statement of Financial Support
This work was supported by National Institutes of Health (NIH) US Public Health Service Program Project Grant P01 HL046925 and the National Center for Research Resources, a part of the NIH, Grant Numbers U54TR001356 and UL1TR000039.
Disclosures:
The authors have the following potential conflicts of interest items to disclose: J.A.W. is a paid consultant for HemoGenix (http://hemogenix.com) and holds a loan agreement with Sysmex Corporation, Kobe, Japan (http://www.sysmex.co.jp/en/index.html) for use of the XE-2100 hematology analyzer in his research lab. D.M.M. is a consultant for MedDay Pharmaceutical.
References
Strauss RG. How I transfuse red blood cells and platelets to infants with the anemia and thrombocytopenia of prematurity. Transfusion 2008;48:209–17.
Linderkamp O, Wu PY, Meiselman HJ. Geometry of neonatal and adult red blood cells. Pediatr Res 1983;17:250–3.
Linderkamp O, Friederichs E, Meiselman HJ. Mechanical and geometrical properties of density-separated neonatal and adult erythrocytes. Pediatr Res 1993;34:688–93.
Landaw SA. Factors that accelerate or retard red blood cell senescence. Blood Cells 1988;14:47–67.
Franco RS. Measurement of red cell lifespan and aging. Transfus Med Hemother 2012;39:302–7.
Widness JA, Kuruvilla DJ, Mock DM, et al. Autologous infant and allogeneic adult red cells demonstrate similar concurrent post-transfusion survival in very low birth weight neonates. J Pediatr 2015;167:1001–6.
Sebring ES, Polesky HF. Fetomaternal hemorrhage: incidence, risk factors, time of occurrence, and clinical effects. Transfusion 1990;30:344–57.
Mock DM, Matthews NI, Zhu S, et al. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion 2011;51:1047–57.
Mock DM, Widness JA, Strauss RG, Franco RS. Posttransfusion red blood cell (RBC) survival determined using biotin-labeled RBCs has distinct advantages over labeling with (51) Cr. Transfusion 2012;52:1596–8.
Mock DM, Widness JA, Veng-Pedersen P, et al. Measurement of posttransfusion red cell survival with the biotin label. Transfus Med Rev 2014;28:114–25.
Trudnowski RJ, Rico RC. Specific gravity of blood and plasma at 4 and 37 degrees C. Clin Chem 1974;20:615–6.
Kuruvilla D, Widness J, Nalbant D, Schmidt R, Mock D, Veng-Pedersen P. A method to evaluate fetal erythropoiesis from postnatal survival of fetal RBCs. AAPS J 2015:1–9.
Arbuckle TE, Wilkins R, Sherman GJ. Birth weight percentiles by gestational age in Canada. Obstet Gynecol 1993;81:39–48.
Freise KJ, Widness JA, Veng-Pedersen P. Erythropoietic response to endogenous erythropoietin in premature very low birth weight infants. J Pharmacol Exp Ther 2010;332:229–37.
Krzyzanski W, Ramakrishnan R, Jusko WJ. Basic pharmacodynamic models for agents that alter production of natural cells. J Pharmacokinet Biopharm 1999;27:467–89.
Krzyzanski W, Woo S, Jusko WJ. Pharmacodynamic models for agents that alter production of natural cells with various distributions of lifespans. J Pharmacokinet Pharmacodyn 2006;33:125–66.
Freise KJ, Widness JA, Schmidt RL, Veng-Pedersen P. Modeling time variant distributions of cellular lifespans: increases in circulating reticulocyte lifespans following double phlebotomies in sheep. J Pharmacokinet Pharmacodyn 2008;35:285–323.
Saleh MI, Nalbant D, Widness JA, Veng-Pedersen P. Population pharmacodynamic analysis of erythropoiesis in preterm infants for determining the anemia treatment potential of erythropoietin. Am J Physiol Regul Integr Comp Physiol 2013;304:R772–81.
Saleh MI, Widness JA, Veng-Pedersen P. Pharmacodynamic analysis of stress erythropoiesis: change in erythropoietin receptor pool size following double phlebotomies in sheep. Biopharm Drug Dispos 2011;32:131–9.
R Core Team 2014 R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
Racine JS. RStudio: a platform-independent IDE for R and Sweave. J Appl Econom 2012;27:167–72.
Veng-Pedersen P. Curve fitting and modeling in pharmacokinetics and some practical experiences with NONLIN and a new program FUNFIT. J Pharmacokinet Biopharm 2015;5:513–31.
Desforges JF, Dawson JP. The anemia of renal failure. AMA Arch Intern Med 1958;101:326–32.
Loge JP, Lange RD, Moore CV. Characterization of the anemia associated with chronic renal insufficiency. Am J Med 1958;24:4–18.
Dziegiel MH, Koldkjaer O, Berkowicz A. Massive antenatal fetomaternal hemorrhage: evidence for long-term survival of fetal red blood cells. Transfusion 2005;45:539–44.
Egberts J, Hardeman MR, Luykx LM. Decreased deformability of donor red blood cells after intrauterine transfusion in the human fetus: possible reason for their reduced life span? Transfusion 2004;44:1231–7.
Strauss RG. Red blood cell transfusion practices in the neonate. Clin Perinatol 1995;22:641–55.
Strauss RG. Transfusion therapy in neonates. Am J Dis Child 1991;145:904–11.
Widness JA, Seward VJ, Kromer IJ, Burmeister LF, Bell EF, Strauss RG. Changing patterns of red blood cell transfusion in very low birth weight infants. J Pediatr 1996;129:680–7.
Acknowledgements
The authors express appreciation to Mark A. Hart for editorial and secretarial assistance. Also appreciated are the many outstanding contributions of Iowa’s neonatal research nurse team including Gretchen Cress, RN, MPH, Karen Johnson, RN, Jin Zhou, RN, and Ruthann Schrock, RN. The gracious help provided by University of Iowa clinical laboratory staff led by Mitchell J. Owen, MT (ASCP), and Mary Capper, MT (ASCP)SH, and overseen by Matthew D. Krasowski, MD, PhD was essential for the success of this study. This work would not have been possible without the permission of the parents of study subjects to allow their infants to participate. Finally, we are grateful to the Sysmex Corporation, Kobe, Japan (http://www.sysmex.co.jp/en/index.html) for the generous loan of the Sysmex XE-2100 automatic hematology analyzer used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuruvilla, D., Widness, J., Nalbant, D. et al. Estimation of adult and neonatal RBC lifespans in anemic neonates using RBCs labeled at several discrete biotin densities. Pediatr Res 81, 905–910 (2017). https://doi.org/10.1038/pr.2017.14
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.14
This article is cited by
-
To transfuse or not transfuse a premature infant: the new complex question
Journal of Perinatology (2019)