Abstract
Background:
The Institute of Medicine (IOM) dietary guidelines for vitamin D are based on limited pediatric data. Our objective was to estimate the dietary vitamin D requirements for maintaining serum 25-hydroxyvitamin D [25(OH)D] concentrations at the various IOM-considered thresholds of vitamin D status (12, 16, and 20 ng/ml) during fall and winter in children.
Methods:
Ninety-six healthy 8- to 14-y-old Pittsburgh-area black and white children enrolled in a randomized, placebo-controlled trial of vitamin D3 1,000 IU daily for 6 mo with baseline and 2-mo follow-up assessments completed during October through April were studied. Vitamin D intake from diet and study supplement adjusted for adherence and serum 25(OH)D were measured.
Results:
The vitamin D intakes needed to maintain serum 25(OH)D concentrations at 12, 16, and 20 ng/ml in 90% of the children were 581, 1,062, and 1543 IU/day, respectively. The estimated vitamin D intakes needed to maintain serum 25(OH)D concentrations at 20 ng/ml in 97.5% of the children was 2,098 IU/day.
Conclusion:
Our data suggest that the current vitamin D recommended dietary allowance (RDA) (600 IU/day) is insufficient to cover the skeletal health needs of at least 50% of black and white children.
Similar content being viewed by others
Main
Vitamin D is an essential nutrient for bone health and calcium homeostasis. Rickets in children and osteomalacia in adults are disorders of poor mineralization of osteoid tissue and are the osteopathic consequence of severe and prolonged vitamin D deficiency (1). Maintaining optimal vitamin D status is critical for the prevention of rickets and the less severe consequences of hypovitaminosis D osteopathy. Sources of vitamin D include, mainly, vitamin D synthesized in the skin from casual sunlight exposure, diet, and dietary supplements (2,3,4). Skin color, season, and latitude of residence influence vitamin D photosynthesis (5,6,7,8,9). The Institute of Medicine (IOM) disregarded the contribution of “photosynthesized vitamin D” while calculating the dietary reference intakes (DRIs) for vitamin D because of the inherent risks of casual sunlight exposure, namely, photo-induced skin damage and skin cancer, and challenges posed by variations of human skin color for quantifying the safe duration of sunlight exposure needed for vitamin D synthesis (10).
The 2011 IOM vitamin D DRI guidelines were based on a risk assessment framework for indicators of bone health such as calcium absorption, bone mineral density, and risk of rickets and osteomalacia in healthy individuals (10). The DRI committee considered circulating concentration of total 25-hydroxyvitamin D [25(OH)D] as a marker of vitamin D status and exposure, and defined concentrations <12 ng/ml as deficient, and ≥20 ng/ml as sufficient, and deemed concentrations of 16 and 20 ng/ml as adequate to meet the skeletal health needs of 50 and 97.5% of the population, respectively (10). The estimated intake of vitamin D required to achieve a serum 25(OH)D concentration of 16 ng/ml in 50% and 20 ng/ml in 97.5% of ≥1-y-old US children, respectively, was 400 and 600 IU/day. These estimates, based on simulations of dose–response data gathered at higher latitudes during winter, were limited by insufficient pediatric data and lack of racially diverse sample of children, and remain to be refined. We utilized data from a randomized, double-blind, placebo-controlled vitamin D3 supplementation trial in black and white children and estimated the dietary vitamin D requirements for maintaining serum 25(OH)D concentrations at the various IOM-considered thresholds of vitamin D status (12, 16, and 20 ng/ml) during fall and winter.
Results
We studied 96 black and white children with baseline and 2-mo follow-up assessments completed during October through April. The mean age of our study children was 11.4 ± 1.9 y. Fifty-two of the children were black and 51 of the children were randomized to the vitamin D supplemented group. Baseline characteristics between vitamin D and placebo group were similar ( Table 1 ). Black children had higher BMI, pubertal status, sunreactive skin type, and melanin index when compared to white children at baseline ( Table 2 ). In addition, at baseline, black children had lower mean 25(OH)D concentrations and higher median serum calcium concentrations than white children ( Table 2 ). Compliance rate by pill count was greater than 80% in the vitamin D and the placebo group and did not differ between the two groups (compliance median (25th, 75th percentile): vitamin D group 0.92 (0.85, 0.99) vs. placebo group 0.92 (0.77, 1), P = 0.74). No child developed hypercalcemia during vitamin D supplementation.
Effects of Vitamin D Supplementation
Effects on mean 25(OH)D concentrations. Baseline 25(OH)D concentrations were not significantly different between the vitamin D and placebo group ( Table 3 ). The 2-mo follow-up 25(OH)D concentrations were higher in children in the supplemented group than in children in the placebo group. The increase in 25(OH)D concentrations in the supplemented children and decrease in 25(OH)D concentrations in children in the placebo group were significant.
Effects on mean calcium, phosphorus, and albumin concentrations. Calcium, phosphorus, and albumin concentrations were not significantly different between the vitamin D and the placebo group at baseline and at the 2-mo follow-up visit ( Table 3 ). The decreases in phosphorus and albumin concentrations in children in the placebo group were significant.
Correlations between change in 25(OH)D concentrations and baseline 25(OH)D concentrations. Lower baseline 25(OH)D concentrations were associated with a higher magnitude of change in 25(OH)D concentrations at the 2-mo follow-up overall (r = −0.33, P = 0.001), and in black children (r = −0.49, P < 0.001) but not in white children (r = 0.05, P = 0.73). Also, the 2 mo changes in 25(OH)D concentrations in the vitamin D supplemented (r = −0.43, P = 0.002) and placebo (r = −0.46, P = 0.002) groups were inversely associated with baseline 25(OH)D concentrations.
Relationship Between Total Vitamin D Intake and 25(OH)D Concentrations
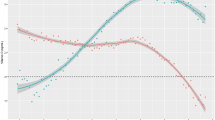
Total vitamin D intake derived from diet and study supplement (vitamin D3 0 or 1,000 IU daily) adjusted for adherence was positively associated with 25(OH)D concentrations during fall and winter (partial r2 = 0.245, P < 0.0001) as shown in Figure 1 . The unadjusted slope of the relation between total vitamin D intake and 25(OH)D concentrations in black and white children was 0.94 ng/ml for each 100 IU/day of vitamin D intake. Race did not modify the association between 25(OH)D concentrations and total dietary vitamin D intake (P = 0.50). Pubertal status was a significant predictor of 25(OH)D concentrations adjusted for total vitamin D intake (P = 0.005). Whereas race (P = 0.26) and BMI (P = 0.31) were not significant predictors of this association.
Relationship between serum 25(OH)D concentrations and total vitamin D (diet + supplement × adherence) intake. The prediction line of this association is shown in bold. The shaded area encompassing the prediction line depicts the 95% confidence limits of the prediction line. The interrupted lines depict the 95% prediction confidence intervals.
The slope of the relation between total vitamin D intake and 25(OH)D concentrations adjusted for race and pubertal status was 0.84 ng/ml for each 100 IU/day of vitamin D intake (R2 = 0.36, P < 0.0001). Our estimated dietary vitamin D requirements for maintaining 25(OH)D concentrations at 12, 16, and 20 ng/ml in 50, 90, 95, and 97.5% of our sample is shown in Table 4 . Our data show that the vitamin D intakes needed to maintain serum 25(OH)D concentrations at 12, 16, and 20 ng/ml in 90% of the children were 581, 1,062, and 1,543 IU/day, respectively. The estimated vitamin D intakes needed to maintain serum 25(OH)D concentrations at 16 ng/ml in 50% of the children was 10.6 IU/day and at 20 ng/ml in 97.5% of the children was 2,098 IU/day.
Discussion
Our results indicate that the current recommended dietary allowance (RDA) for vitamin D may be inadequate in children residing in higher latitudes during winter. The IOM has suggested that maintaining 25(OH)D concentrations ≥20 ng/ml as sufficient for optimal skeletal health in children and an intake of 600 IU of vitamin D/day as the RDA for maintaining 25(OH)D concentrations at 20 ng/ml in 97.5% of children ≥ 1 y of age (10). According to our findings, the current vitamin D RDA (600 IU/day) is insufficient to cover the skeletal health needs of at least 50% of black and white children.
The IOM vitamin D DRI committee had deemed 25(OH)D concentrations of 16 ng/ml as the median value for maintaining optimal skeletal health—concentration above which will be adequate for 50% of the population and concentration below which will be inadequate for 50% of the population (10). The committee set 12 and 20 ng/ml as the lower and upper limits of 25(OH)D requirements for meeting the skeletal health needs, with the presumption that 25(OH)D concentrations of 20 ng/ml would be sufficient to cover the needs of 97.5% of the population and <12 ng/ml would be associated with vitamin D deficiency (10). The committee examined dose–response data from nine vitamin D intervention studies conducted during winter (minimal UV-B exposure) in 6- to >60-y-old individuals living in higher latitudes in northern Europe (>49.5°N) and Antarctica (78°S) and formulated a regression equation for estimating the response of 25(OH)D concentrations to total vitamin D intake (10). The IOM committee estimated vitamin D intake required to achieve a 25(OH)D concentration of 16 ng/ml in 50% of the population (estimated average requirement (EAR)) and 20 ng/ml in 97.5% of the population (RDA) were 400 and 600 IU/day, respectively (10). Whereas, in our study, the EAR was 10.6 IU/day and RDA was 2,098 IU/day. Our considerably lower EAR can be explained by likely the contributions of summertime sun exposure to serum 25(OH)D concentrations, as our study was conducted at a lower latitude (40.4°N and 40.8°N) than the studies considered by IOM. The differences in the estimates can be attributed to the differences in the regression models and cohort characteristics. The IOM estimates were based on a curvilinear fit model derived using pooled data from 6- to >60-y-old individuals, whereas our estimates were based on a linear regression model derived using data from a single site study of 8- to 14-y-old black and white children, and half of our sample comprised of black children.
In this study, supplementation with vitamin D3 1,000 IU daily for 2 mo during October through April was safe and effective. Mean 25(OH)D concentrations increased significantly in the supplemented children and decreased significantly in the placebo group at 2 mo. The decrease in 25(OH)D concentrations in the placebo group reflect the influence of season on vitamin D status of children living in higher latitudes during fall and winter, and validate that our assessments were done during periods of minimal UV-B light exposure. The decrease in phosphorus and albumin at 2 mo in the placebo group was not clinically relevant. The magnitude of change in 25(OH)D concentrations at the 2-mo follow-up was inversely associated with baseline 25(OH)D concentrations in black but not in white children mainly because black children had significantly lower basal 25(OH)D concentrations than white children (11,12).
In this study, pubertal status was a significant predictor of 25(OH)D concentrations. This finding is consistent with our previous report examining the predictors of 25(OH)D concentrations in 8- to 18-y-old black and white children (13). We speculate that this is primarily due to the fact that adolescent children have higher rates of vitamin D deficiency and lower intakes of dietary vitamin D when compared to preadolescent children (14).
Data regarding estimations of dietary requirements of vitamin D in children are limited. Cashman et al. (15) have reported a higher slope for the relation between vitamin D intake and 25(OH)D concentration (2.43 ng/ml per 100 IU of vitamin D3) during winter in 144 white, healthy adolescent Finnish (latitude: 55°N) and Danish (latitude 60°N) girls, and their estimated EAR and RDA were 200 and 744 IU/day, respectively. Stratified dosing (placebo or 200 or 400 IU/day) yielding a tighter and a well-correlated dose–response curve, a sample comprising of only Caucasian adolescent girls, higher basal 25(OH)D concentration (22.6 ± 5.6 ng/ml), and lack of influence of basal 25(OH)D on the responsive changes in 25(OH)D could explain the contrast in the findings between the present study and their report.
Our findings highlight the relevance of the contributions of summertime sun exposure for maintenance of 25(OH)D concentrations during winter in children living in the Northeastern parts of US (13,16)—25(OH)D concentrations of 16 ng/ml was maintained in 50% of our sample without any or negligible intake of dietary vitamin D. The estimated daily intake of vitamin D required to maintain 25(OH)D concentrations at 16 and 20 ng/ml in 50% of our study cohort was 10.6 and 489 IU respectively. However, it’s apparent from our data that the current IOM-set RDA of 600 IU/day may be inadequate. Our estimated intakes of vitamin D needed to maintain 25(OH)D concentrations at 12, 16, and 20 ng/ml in 97.5% of our study cohort during winter were 1,137, 1,618, and 2,098 IU/day, respectively. Our findings are relevant for determining the vitamin D requirements of children residing in other areas of the world with comparable latitudes (above 40°N (e.g., Madrid, Spain) or below 40°S).
Guidelines regarding dietary vitamin D requirements and concentrations of 25(OH)D deemed as essential for optimal skeletal health remain controversial and lack consensus at a global level. The Endocrine Society in the US has defined 25(OH)D threshold concentrations for vitamin D deficiency, insufficiency, and sufficiency as <20, 20 – <30, and ≥30 ng/ml, respectively; and recommended a vitamin D intake of 600–1,000 IU/day in 1- to 18-y-old children for the prevention of vitamin D deficiency and suggested a vitamin D intake of 1,000 IU/day to consistently maintain their 25(OH)D concentrations above 30 ng/ml (17). Vitamin D guidelines also vary widely in European countries (18). Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden) recommend a vitamin D intake of 400 IU/day for infants and children to achieve a target serum 25(OH)D concentration of ≥20 ng/ml (19). Children residing in Germany, Austria, and Switzerland have been recommended to meet a daily vitamin D intake of 800 IU/day for maintaining a target 25(OH)D concentration of ≥20 ng/ml (18). Central European children are recommended a vitamin D intake of 600–1,000 IU/day to maintain a target 25(OH)D concentrations of 30–50 ng/ml (20). Given the variability in the vitamin D recommendations, a unifying policy guideline for addressing the prevention of vitamin D deficiency at global level is warranted (21).
Limitations of our study include short duration of vitamin D intervention with single instead of multiple doses, lack of information regarding travel to sunny location during the intervention period, and the presumption that the association between vitamin D intake and change in 25(OH)D concentrations is linear. Recent studies have highlighted the relevance of dietary 25(OH)D from animal foods as an undocumented food source of vitamin D (22). Vitamin D content of certain animal foods based on their potency-adjusted 25(OH)D content were higher and increased by 68 to 116 IU (23). Incorporation of potency-adjusted 25(OH)D content in our estimates of basal dietary intake of vitamin D could have reduced some of the incongruence between the estimated vitamin D intake and serum 25(OH)D concentrations, namely, our considerably lower EAR of 10.6 IU/day for maintaining 25(OH)D concentrations of 16 ng/ml in 50% of our study population. Representation of black children and contribution to the limited literature pertaining to the estimation of dietary vitamin D requirements in children are strengths of our study.
In conclusion, 25(OH)D concentration is positively associated with total vitamin D intake in black and white children during winter. Pubertal status is a significant predictor of 25(OH)D adjusting for vitamin D intake. Vitamin D intake required for maintaining 25(OH)D concentrations at 20 ng/ml in 97.5% of black and white children, adjusting for race and pubertal status, was threefold higher (≈2,000 IU/day) than the current RDA (600 IU/day). Our findings suggest that future studies are warranted to refine the current IOM suggested RDA for vitamin D in children.
Methods
Study Design and Participants
We conducted a secondary analysis in a subset of subjects enrolled in a randomized, double-blind, placebo-controlled trial (Clinicaltrials.gov identifier: NCT00732758) that examined the effects of supplementation with vitamin D3 1,000 IU daily for 6 mo on serum 25(OH)D, parathyroid hormone, and markers of bone turnover in healthy 8- to 14-y-old black and white children from Pittsburgh (latitude: 40.4°N) and Kittanning (40.8°N), Pennsylvania. Children not receiving vitamin supplements entered the trial during October through March of 2008 through 2011. Details and findings of the primary study have been reported (24). Our subjects for this study were children with baseline and 2-mo follow-up evaluations completed during October through April, a period of reduced solar ultraviolet-B (UV-B) radiation. We chose to examine the 2 mo rather than the 6-mo outcomes mainly because majority of the 6-mo follow-up visits (89%) were completed during late spring and summer months (May through September). This strategy for subject selection eliminated the confounding effects of sun exposure on the relationship between dietary vitamin D and serum 25(OH)D. The clinical trial was approved by the University of Pittsburgh Institutional Review Board. Parental consent and subjects’ assent were obtained prior to initiation of study procedures.
Study Intervention
Children were randomized by race to receive a vitamin D3 1,000 IU or placebo tablet once daily in a 1:1 ratio. Both the tablets were manufactured by Douglas Laboratories (Pittsburgh, PA), and were similar in color and appearance, and were dispensed masked in identical containers. Study medications were manufactured and supplied in two batches. The average vitamin D3 content of the vitamin D3 tablet, analyzed as described before (25), was ≈1129 IU in the first batch around the mid-point of its shelf-life, and 1,140 IU in the second batch at the end of the trial.
Study Measurements
Anthropometry, skin color, dietary vitamin D and calcium, pubertal status, biochemical measurements, and compliance. Our baseline study measurements included height, weight, calculated BMI, skin color, pubertal status, and dietary intake of vitamin D and calcium intake. We assessed skin color by parent-reported sunreactive skin type (26,27) and forehead melanin index using DSM II Colormeter, a hand-held dermatospectrophotometer (Cortex Technology, Hadsund, Denmark). We estimated pubertal status by physical examination (28,29) and dietary intake of vitamin D and calcium using a validated food frequency questionnaire (30,31). Our biochemical measurements for this analysis included serum calcium, phosphorus, albumin, and 25(OH)D collected at baseline and 2-mo follow-up. Serum 25(OH)D was assayed using liquid chromatography-tandem mass spectrometry as described before (24,32). We assessed compliance by pill count at the 2-mo follow-up.
Statistical Analysis
We conducted a secondary analysis from a randomized, placebo-controlled vitamin D trial with a sample size of 157 children (24). Only children with baseline and 2-mo follow-up visits completed between October and April and no missing adherence data were included in this analysis. Therefore, the sample size for this analysis was decreased to 96 children. This sample size was not determined prior to the study.
We compared the baseline demographic, clinical, and dietary characteristics across race (black vs. white children) and treatment (vitamin D-supplemented vs. placebo) groups using two-sample t-tests and chi-square analyses. We used Wilcoxon Rank Sum tests to compare the medians between the respective race and treatment groups when the data were skewed. We used analysis of covariance to compare 2-mo 25(OH)D, calcium, phosphorus, and albumin between the vitamin D and placebo groups controlling for the corresponding baseline measure. We tested within group changes of the measures using paired t-tests. We estimated the correlation between baseline 25(OH)D and changes in 25(OH)D using Pearson correlation coefficient. To estimate the required vitamin D intake for achieving certain concentrations of serum 25(OH)D, we used methods similar to Cashman et al. (15) by modeling the association between 25(OH)D as a function of dietary vitamin D (assigned group dose × adherence + dietary vitamin D intake at baseline) adjusting for race and Tanner stage. We tested if the association between dietary vitamin D and 25(OH)D was different for black and white children using an interaction term in the linear regression model. From the final regression model, we estimated the dietary intake of vitamin D that would correspond to having a percent (50, 90, 95, 97.5) of the population maintain serum 25(OH)D concentrations of ≥12, ≥16, and ≥20 ng/ml. The 95% confidence intervals were calculated using bootstrap based on 10,000 replications. All tests were two sided (α = 0.05) and analyses were conducted in SAS Enterprise Guide 6.1 (SAS Institute, Cary, NC).
Statement of Financial Support
This work was supported by the following grants: National Institutes of Health grants (Bethesda, MD, USA) K23HD052550 (KR), R01HL112985 (KR and CGM), K24DK062895 (SLG), P30 AG024827 (SLG), and UL1 RR024153 (University of Pittsburgh Clinical and Translational Research Center & Pediatric PittNet); and Children’s Hospital of Pittsburgh Research Advisory Committee seed grant (KR). Clinicaltrials.gov identifier: NCT00732758
Disclosure
Authors have no conflicts of interest pertaining to this manuscript.
References
Heaney RP. Lessons for nutritional science from vitamin D. Am J Clin Nutr 1999;69:825–6.
Haddad JG Jr, Hahn TJ. Natural and synthetic sources of circulating 25-hydroxyvitamin D in man. Nature 1973;244:515–7.
Lawson DE, Paul AA, Black AE, Cole TJ, Mandal AR, Davie M. Relative contributions of diet and sunlight to vitamin D state in the elderly. Br Med J 1979;2:303–5.
Poskitt EM, Cole TJ, Lawson DE. Diet, sunlight, and 25-hydroxy vitamin D in healthy children and adults. Br Med J 1979;1:221–3.
Holick MF, MacLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science 1980;210:203–5.
McLaughlin M, Raggatt PR, Fairney A, Brown DJ, Lester E, Wills MR. Seasonal variations in serum 25-hydroxycholecalciferol in healthy people. Lancet 1974;1:536–8.
Norman AW. Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. Am J Clin Nutr 1998;67:1108–10.
Stamp TC, Round JM. Seasonal changes in human plasma levels of 25-hydroxyvitamin D. Nature 1974;247:563–5.
Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988;67:373–8.
Institute of Medicine. Dietary Reference Intake for calcium and vitamin D. Washington, DC: The National Academy Press; 2011.
Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:1080S–6S.
Ng K, Scott JB, Drake BF, et al. Dose response to vitamin D supplementation in African Americans: results of a 4-arm, randomized, placebo-controlled trial. Am J Clin Nutr 2014;99:587–98.
Rajakumar K, de las Heras J, Chen TC, Lee S, Holick MF, Arslanian SA. Vitamin D status, adiposity, and lipids in black American and Caucasian children. J Clin Endocrinol Metab 2011;96:1560–7.
Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr 2008;88:558S–64S.
Cashman KD, FitzGerald AP, Viljakainen HT, et al. Estimation of the dietary requirement for vitamin D in healthy adolescent white girls. Am J Clin Nutr 2011;93:549–55.
Rajakumar K, Holick MF, Jeong K, et al. Impact of season and diet on vitamin D status of African American and Caucasian children. Clin Pediatr (Phila) 2011;50:493–502.
Holick MF, Binkley NC, Bischoff-Ferrari HA, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30.
Spiro A, Buttriss JL. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr Bull 2014;39:322–50.
Nordic Council of Ministers. Nordic Nutrition Recommendation 2012. Integrating Nutrition and Physical Activity. 5th edn. Norden: Copenhagen, Denmark, 2012. https://www.norden.org/en/theme/nordic-nutrition-recommendation/nordic-nutrition-recommendations-2012. 2015.
Płudowski P, Karczmarewicz E, Bayer M, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe - recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol 2013;64:319–27.
Schoenmakers I, Pettifor JM, Pena-Rosas JP, et al. Prevention and consequences of vitamin D deficiency in pregnant and lactating women and children: A symposium to prioritise vitamin D on the global agenda. J Steroid Biochem Mol Biol 2015 Nov 10. pii: S0960-0760(15)30130-8. doi: 10.1016/j.jsbmb.2015.11.004 (Epub ahead of print).
Heaney RP, Armas LA, French C. All-source basal vitamin D inputs are greater than previously thought and cutaneous inputs are smaller. J Nutr 2013;143:571–5.
Taylor CL, Patterson KY, Roseland JM, et al. Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. J Nutr 2014;144:654–9.
Rajakumar K, Moore CG, Yabes J, et al. Effect of vitamin D3 supplementation in black and in white children: a randomized, placebo-controlled trial. J Clin Endocrinol Metab 2015;100:3183–92.
Chen TC, Turner AK, Holick MF. A method for the determination of the circulating concentration of vitamin D. J Nutr Biochem 1990;1:272–6.
Jimbow K, Fitzpatrick TB, Wick MM. Biochemistry and physiology of melanin pigmentation. In: Goldsmith LA, ed. Phyisology, Biochemistry, and Molecular Biology of the Skin. New York, NY: Oxford University Press; 1991:893–909.
Pathak M, Jimbow K, Szabo G, Fitzpatrick TB. Sunlight and melanin pigmentation. In: Smith K, ed. Photochemical and Photobiological Reviews. New York, NY: Plenum Press; 1976:211–39.
Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303.
Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23.
Rockett HR, Breitenbach M, Frazier AL, et al. Validation of a youth/adolescent food frequency questionnaire. Prev Med 1997;26:808–16.
Rockett HR, Wolf AM, Colditz GA. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. J Am Diet Assoc 1995;95:336–40.
Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 2005;90:3215–24.
Acknowledgements
K.R., C.G.M., and J.Y.: conceptualized and designed the study; K.R., F.O., and M.A.H.: conducted the research and obtained the data; C.G.M. and J.Y.: performed the statistical analysis; K.R., C.G.M., S.L.G., M.F.H., and J.Y.: interpreted the data; M.F.H.: performed the 25(OH)D assay; K.R., C.G.M., J.Y., and D.C.: drafted the manuscript; All authors: critically revised the manuscript; K.R.: secured funding and supervised the study. K.R. had full access to all of the data in this study and takes responsibility for the integrity and accuracy of this work as a whole from inception to publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Rajakumar, K., Moore, C., Yabes, J. et al. Estimations of dietary vitamin D requirements in black and white children. Pediatr Res 80, 14–20 (2016). https://doi.org/10.1038/pr.2016.46
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2016.46
This article is cited by
-
Individual participant data (IPD)-level meta-analysis of randomised controlled trials among dark-skinned populations to estimate the dietary requirement for vitamin D
Systematic Reviews (2019)
-
A systematic review and meta-analysis of the response of serum 25-hydroxyvitamin D concentration to vitamin D supplementation from RCTs from around the globe
European Journal of Clinical Nutrition (2019)
-
Differences in the dietary requirement for vitamin D among Caucasian and East African women at Northern latitude
European Journal of Nutrition (2019)
-
Supplementation with Three Different Daily Doses of Vitamin D3 in Healthy Pre-pubertal School Girls: A Cluster Randomized Trial
Indian Pediatrics (2018)
-
The effect of vitamin D on COPD exacerbation: a double blind randomized placebo-controlled parallel clinical trial
Journal of Diabetes & Metabolic Disorders (2015)