Abstract
Background:
Clustering of cardiovascular disease (CVD) risk factors has been found in children as young as 9 y of age. However, the stability of this clustering over the course of childhood has yet to be determined. The purpose of this study was to determine the tracking of clustered CVD risk from young school age through adolescence and to examine differences in tracking between levels of overweight/obesity and cardiorespiratory fitness (VO2peak).
Methods:
Beginning at 6 y, children (n = 434) were measured three times in 7 y. Anthropometrics, blood pressure, and VO2peak were measured. Fasting blood samples were analyzed for CVD risk factors. A clustered risk score (z-score) was constructed by adding sex-specific z-scores for blood pressure, homeostatic model assessment (HOMA-IR), triglyceride (TG), skinfolds, and negative values of high-density lipoprotein cholesterol (HDLc) and VO2peak.
Results:
Significant tracking coefficients were found between clustered z-score at all time intervals (r = 0.514, 0.559, and 0.381 between ages 6–9, 9–13, and 6–13 y, respectively, all P < 0.0001). Tracking was higher for low-fit children, whereas no clear pattern was found for different levels of body fat.
Conclusion:
We found that clustered z-score is a fairly stable characteristic through childhood. Implementation of preventive strategies could therefore start at early school age.
Similar content being viewed by others
Main
High risk for future cardiovascular disease (CVD) is difficult to define in children, given that no hard end points, such as manifest disease or death, have yet occurred. Clustering of individual risk factors in the same individual has been suggested as a good method to assess CVD risk level in apparently healthy children, given that it describes a state in which several risk factors are high simultaneously in the same individual (1). Earlier studies from our group found no clustering of CVD risk factors in 6-y-old children in this cohort, whereas at the age of 9 y, three or more CVD risk factors were found in 3.33 times as many participants than expected, which corresponded to 13.8% of the population (2). However, clustering of CVD risk factors in children is of most interest if it is a stable characteristic. Tracking is a term used to describe the development of a characteristic over time and involves both the longitudinal stability of the variable and the ability of one measurement to predict the value of a following measurement(s) (3). A recent review of the literature regarding tracking of CVD risk factors from childhood to adulthood concluded that despite the differences in methodology, studies have consistently found tracking of cardiometabolic risk factor clustering from childhood or adolescence to adulthood (4). However, the researchers concluded that the shorter-term stability of clustered risk factors over the course of childhood and adolescence has yet to be elucidated. Knowledge about this stability is potentially important, given that it might demonstrate whether there is an optimal time point in childhood to measure health variables and possibly initiate interventions. Furthermore, clustering of CVD risk factors in children has been found to be associated with lifestyle factors such as obesity (1,5,6,7,8) and cardiorespiratory fitness (VO2peak) (1,7,8,9). To our knowledge, only one study in youth has examined the effect of obesity on tracking of clustered risk (10), and no studies have looked at the effect of VO2peak. Therefore, the aim of this study was to evaluate tracking of clustered CVD risk in youth using three time points from 6 to 13 y of age and to examine how different levels of overweight/obesity and VO2peak affect the precision of tracking.
Results
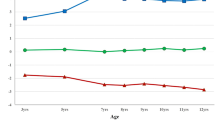
The general characteristics of the sample at the ages of 6, 9, and 13 y are presented in Table 1 . BMI, sum of the four skinfolds (S4SF), systolic blood pressure, and homeostatic model assessment (HOMA-IR) increased with age. VO2peak increased from age 6 to age 9 y, triglycerides (TGs) increased from age 9 to age 13 y, and high-density lipoprotein cholesterol (HDLc) increased from age 6 to age 9 y and then decreased from age 9 to age 13 y. Tracking coefficients between clustered z-score at age 6–9 y, 9–13 y, and 6–13 y are presented in Table 2 . Moderate correlations were found between clustered z-score at the ages of 6–9 y and 9–13 y, whereas the coefficient between the ages of 6 and 13 y was somewhat weaker. These coefficients did not change significantly when analyses were performed for each sex separately (data not shown). For the single risk factors, highest tracking coefficients were seen for S4SF, followed by systolic blood pressure and HDLc. Tracking coefficients for HOMA-IR and TG were low to moderate. The tracking coefficients for VO2peak were high from 6 to 9 y and 9 to 13 y, but low from 6 to 13 y.
Logistic regression showed that when children with moderate- and high-risk clustered z-score at the first time point were compared with those with low-risk clustered z-score, they had a 6.1 and 21.2 times greater risk, respectively, of having a clustered z-score above 1 SD at the second time point between the ages of 6 and 9 y, 4.5 and 30.8 times greater risk, respectively, between the ages of 9 and 13 y, and 2.3 and 4.9 times greater risk, respectively, between the ages of 6 and 13 y ( Table 3 ).
When children were grouped on the basis of their level of VO2peak, tracking coefficients were higher in the first tertile (least fit) at all three time intervals (r values: 0.50–0.67). The coefficients were strong in the first and second tertiles and low in the third tertile (r values: 0.25–0.39). When children were grouped on the basis of S4SF, the picture was not as clear as that for VO2peak. We found lowest tracking coefficients in the first tertile (least fat) at all three time intervals. Between the ages 6 and 9 y, there was no significant coefficient in the first tertile (r value: 0.068), a strong coefficient in the second tertile (r value: 0.499), and moderate coefficient in the third tertile (r value: 0.34). Between the ages of 6 and 13 y, the coefficients were weak in all tertiles (nonsignificant in the first) (r values: 0.20, 0.31, and 0.23 in first, second, and third tertiles, respectively). Between the ages of 9 and 13 y, the coefficients were weak in the first and the second tertiles (r values: 0.32 and 0.30) and stronger in the third tertile (r value: 0.49).
Discussion
This study examined the short-term tracking of a clustering of CVD risk factors in a normal youth population from early school age to the beginning of adolescence. We found moderate to high levels of tracking between all the measured time points, and the risk of having a high sum of z-score at a second time point was between 2.3 and 30.8 times higher for children having a high sum of z-score at the first time point. Similarly, the only other study examining the stability of clustered CVD risk factors from childhood (age: 9 y) to adolescence (age: 15 y) in a sample of Swedish and Estonian children found moderate overall tracking coefficients for boys and girls, respectively (11). Moreover, studies looking at clustered CVD risk factor tracking from childhood or adolescence to adulthood displayed similar results (12,13,14,15). Given that CVD risk factors did not cluster at 6 y in this cohort (16), it was unexpected to find a high degree of tracking between the ages of 6–9 y and 6–13 y. The results indicate that a relatively high level of several CVD risk factors, even though they were independently distributed (no clustering), at this low age, is predictive of future high metabolic risk. Juhola et al. (17), using data from the Cardiovascular Risk in Young Finns Study, investigated the sensitivity and specificity rates for predicting abnormal CVD risk factors in an adult from values obtained at age 3, 6, 9, 12, 15, and 18 y. They found that the obesity and blood pressure values at all childhood ages were predictive of the adult values. For HDLc, there was no difference between age groups for females, but for males, HDLc levels at ages 6, 9, and 12 y were most predictive of adult values, as compared with ages 3, 15, and 18 y. Taken together, these results suggest that CVD risk factor levels are fairly stable from early childhood to adulthood. We did not find any sex differences in the tracking of clustered CVD risk (data not shown), which is similar to the results from some (11,12), but not all, studies (18).
In the current study, all of the single risk factors included in the summed z-score had a positive tracking coefficient ( Table 2 ), which means that they all contributed to the tracking coefficient for the sum of z-score. However, tracking coefficients of single risk factors varied from low to high, whereas the tracking coefficients for the clustered z-score varied from moderate to high. On the basis of these results, there is no indication of a much higher tracking of a clustered risk score, as compared with the single risk factors. We do not have any explanation for this, given that we expected to find a substantially higher tracking of the clustered z-score because of the advantages of this score (see below). Moreover, Bao et al. (15) found that tracking expressed as an interage correlation was higher for a clustered risk score (r = 0.64) as compared with the single risk factors (r = 0.34–0.57); conversely, others did not find this difference between individual and clustered risk factors (12,13). The differences between the studies could be related to the samples used for each study. We used relatively healthy Danish children, whereas the comparative studies used North American children.
Clustering of CVD risk factors has been found to be related to VO2peak in cross-sectional studies in youth (1,7,9,14,19). We analyzed the stability of the summed CVD risk factors within each tertile of VO2peak and found that a lower baseline VO2peak level was associated with a higher tracking coefficient of clustered z-score; this was consistent for all time intervals. This implies that within the least fit group, the stability of metabolic health is greater as compared with that within the most fit group. Conjecture suggests that it takes more effort to positively change health-affecting behaviors (e.g., lose weight or increase physical activity and fitness) as compared with changing behavior in a way that negatively influences health (e.g., gaining fat and decreasing physical activity). To our knowledge, no other studies have examined the effect of VO2peak level on the degree of tracking of clustered CVD risk factors in this age group, and our results should, therefore, be verified in other cohorts.
In this study, when children were grouped on the basis of their S4SF, no clear picture was found between groups in tracking of clustered CVD risk factors. This could suggest that the level of fatness may not be pivotal for the stability of CVD risk factor clustering over time in a normal youth population. This is not to say that overweight does not play an important role in the development of clustering of risk factors, a fact that has been demonstrated in several studies. In cross-sectional studies, overweight and obesity have consistently been found to be related to clustering of CVD risk factors in children and adolescents (1,5,6,7), and convincing evidence suggests that overweight and obesity tracks from childhood into adulthood (20). Furthermore, some studies have found that overweight or obesity in childhood is associated with increased risk of later development of CVD risk factor clustering (21,22). However, in one of these studies, the association disappeared when fatness was removed from the clustered risk score. Thus, the authors concluded that the effect of fatness in childhood on the cluster of CVD risk factors in adolescence is a result of adiposity tracking (21). In support of this, a recent review concluded that there is not much evidence for childhood obesity to be an independent risk factor for adult CVD risk, because the relationship is attenuated or no longer present when adjusting for adult obesity (23). Likewise, Chen et al. (10) demonstrated that longitudinal changes in clustering of CVD risk factors assessed by incremental area (area under the curve) were decreased by ~50%, when adjusted for BMI. These results suggest that fatness is an important part of a causal chain leading to clustered CVD risk. It is, however, not possible to conclude anything about causation; it could be that fatness is an intermediate, and adjusting for an intermediate may remove the associations between the investigated exposure and the outcome.
The strength of this study is the relatively large cohort followed for a 7-y period with three measurements from early school age to the beginning of adolescence. The quality of included measurements, e.g., the direct measurement of VO2peak, is a strength of the study. Moreover, the inclusion of the clustered z-score strengthens the study. As stated earlier, this type of outcome may be a better indicator of health in apparently healthy children and adolescents, as compared with the individual risk factors, as it uses all available information (no cut points, but a continuous score). Furthermore, a clustered score can to some extent compensate for the day-to-day fluctuations in the individual risk factors, and it is not as sensitive to measurement errors. However, this method also has some limitations. First, no consensus exists regarding which risk factors to include in the score or how to define “high risk.” Therefore, the risk is specific to the study sample only and is not based on a biologic measure. Second, the clustered z-score is based on the assumption that all included individual CVD risk factors are equally important in defining CVD risk, which may not be the case. However, there is no evidence suggesting how risk factors should be weighed, and we may never get that in children, because CVD is not usually manifested in children. Another limitation of this study is the relatively lean and healthy population studied, which could limit the generalizability of the results especially to more overweight and metabolically impaired populations. However, as mentioned, we have previously found that in this cohort, CVD risk factors cluster in ~14% of the children from the age of 9 y and that this cluster is related to VO2peak and fatness (2). Another factor possibly influencing our results is the dropout of the study, which has been described in detail elsewhere (11). Briefly, it was shown that there were no significant differences in 6-y values of parental income, BMI z-score, waist circumference, or VO2peak between children who participated at age 9 y and children who dropped out. However, children participating at age 13 y came from families with a higher socioeconomic status, on the basis of parental income at baseline, and had significantly lower baseline BMI z-scores and waist circumference and a higher baseline VO2peak as compared with the children who dropped out of the study. This is also apparent from the results in BMI z-scores seen in Table 1 , in which the value is lower at age 13 y as compared with ages 6 and 9 y, implying that the fattest participants have dropped out. Furthermore, the dropout is larger in some variables, e.g., VO2peak and blood variables, because of the strict inclusion criteria and invasive character of these measurements. Therefore, the number of subjects included in the analysis of these variables and the clustered z-score is considerably less as compared with, e.g., S4SF. We did not include any measure of sexual maturation in our analysis. However, given that maturation is related to many of the CVD risk factors, e.g., subjects become transiently insulin resistant in mid-puberty, this could possibly affect our results. We therefore ran all analyses as partial correlations adjusting for maturation and this did not change our results significantly. Finally, the size of the tracking coefficient is highly dependent on the reproducibility of the measurement and its error variation. In that regard, S4SF is easier to reproduce as compared with some of the other variables measured, e.g., VO2peak. Moreover, as described above, the clustered z-score is easier to reproduce than some of the individual risk factors.
In conclusion, moderate to strong tracking was found for clustered z-score in a normal pediatric population measured three times from age 6 to age 13 y. Furthermore, children with a higher clustered z-score at first measurement had an increased risk of having a clustered z-score above 1 SD at the second measurement. This means that a high level in several CVD risk factors in early school age is already predictive for the development of the clustering of CVD risk factors seen in the older age groups. Tracking of clustered z-score differed between tertiles of fitness, with children in the lowest fitness group displaying the highest tracking coefficients. The picture was not as clear for tertiles of fatness. We believe that the results of this study have important clinical implications, given that they show stability of clustering of CVD risk factors across school age and point to implementation of preventive strategies starting in early childhood.
Methods
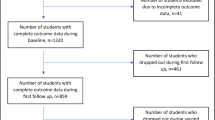
Participants in The Copenhagen School Child Intervention Study, which was started in 2001, were the subjects of this study, which included all children attending kindergarten class in two communities in the area of Copenhagen (46 kindergarten classes in 18 public schools). Written informed consent was obtained from the parents/guardians of 706 children (69% of the population), and 696 actually participated in the study at baseline. Following the intervention, the children were retested in 2004/2005 at the age of 9 y and followed up again in 2008 at the age of 13 y. The study has been described in detail elsewhere (24,25,26). In the current analysis, we included all children with complete measures of CVD risk factors from at least two of the three time points (n = 434). The study was approved by the ethical committee, University of Copenhagen.
Because the complete methodology has been previously published, the methodology presented here includes only those variables of interest. Height and body weight were measured according to standard procedures. BMI was calculated (kg/m2), and BMI z-scores were computed on the basis of the World Health Organization recommendations (27). Bicipital, tricipital, subscapular, and suprailiac skinfolds were measured using standard procedures with a Harpenden skinfold caliper (Harpenden, West Sussex, UK). The S4SF was used as a measure of body fatness (28).
VO2peak was assessed using a continuous running protocol on a treadmill until exhaustion. VO2peak was measured directly on an AMIS 2001 Cardiopulmonary Function Test System (Innovision, Odense, Denmark) at ages 6 and 9 y and using the COSMED K4b2 portable metabolic system (COSMED, Rome, Italy) at age 13 y. Both systems provide valid measures of VO2 when validated against the Douglas bag method (29,30). Detailed criteria for an accepted test have been reported earlier (25). Blood pressure was measured in the sitting position after 15 min of rest with a Dinamap XL vital signs blood pressure monitor (Critikron, Tampa, FL) using appropriately sized cuffs. The mean of the last three of five measurements taken over 10 min was used for the analysis.
Fasting blood samples were taken between 08:00 and 09:30 h, and glucose was analyzed immediately (Hemocue, Ängelholm, Sweden). The remainders of the samples were centrifuged, and plasma was aliquoted within 30 min, kept at −20 °C, and later stored at −80 °C until analyzed. Insulin was analyzed spectrophotometrically using an enzyme-linked immunosorbent assay, code no. K6219 (DAKO Insulin, Glostrup, Denmark). Insulin resistance was estimated according to HOMA-IR as glucose (mmol/1) multiplied by insulin (mU/1) divided by 22.5 (31). Blood lipids were analyzed on a COBAS FARA (Roche, Switzerland) using spectrophotometry (ABX diagnostics, Montpellier, France).
Statistical Analysis
A composite CVD risk score (clustered z-score) was constructed by adding the sex-specific z-scores for systolic blood pressure, HOMA-IR, TG, and S4SF, and the negative z-scores for HDLc and VO2peak. Rationale for choosing these variables for the clustered z-score has been described elsewhere (32). In analysis with stratification for S4SF, S4SF was removed from the clustered z-score and in analysis with stratification for VO2peak, this variable was removed from the clustered z-score.
No differences in the clustered z-score between the intervention and control groups of the Copenhagen School Child Intervention Study were evident (24), so the groups were pooled for all analyses. For descriptive purposes, means and SDs for all variables at ages 6, 9, and 13 y were computed. BMI, S4SF, HOMA-IR, and TG were positively skewed and therefore transformed (natural log) for the analyses. Tracking coefficients were calculated between the clustered z-score at the ages of 6–9, 6–13, and 9–13 y using Pearson correlations (3). This was also performed for each of the CVD risk factors to determine which factors were the most important in the tracking of the clustered z-score. High-risk cases were defined as having a clustered z-score above 1 SD, corresponding to ~16, 14, and 12% of the population at ages 6, 9, and 13 y, respectively. This choice of assigning high risk was based on an earlier study by Andersen et al. (32), indicating that a clustering of CVD risk factors, defined as being in the least favorable quartile of four or more risk factors, was found in ~11% of a normal European pediatric population. This group was characterized by the fact that risk factors were not independently distributed, or in other words, they clustered. The authors of that study defined 1 SD above the mean in summed z-score to be at risk, which was higher than the actual calculation of 16% (32). We chose to use the same risk cut point, even though it is arbitrary, given that no evidence exists regarding a biologic cut point over which CVD risk increases for these age groups. Logistic regression analysis was performed and the odds ratios among children were assessed in three different risk categories—low risk (< median z-score), moderate risk (median to 1 SD), and high risk (≥1 SD)—at one time point for being at high risk at the second time point. Finally, to evaluate the influence of VO2peak and fatness (S4SF), children were grouped in tertiles on the basis of these variables, and clustered z-score (without VO2peak or S4SF, respectively) tracking coefficients were calculated within each tertile using Pearson correlations. All analyses were performed using the Statistical Package for the Social Sciences version 19 (SPSS, Chicago, IL).
Statement of Financial Support
This study was funded by the Danish Heart Association and Trygfonden (Copenhagen, Denmark).
References
Andersen LB, Sardinha LB, Froberg K, Riddoch CJ, Page AS, Anderssen SA . Fitness, fatness and clustering of cardiovascular risk factors in children from Denmark, Estonia and Portugal: the European Youth Heart Study. Int J Pediatr Obes 2008;3:Suppl 1:58–66.
Andersen LB, Bugge A, Dencker M, Eiberg S, El-Naaman B . The association between physical activity, physical fitness and development of metabolic disorders. Int J Pediatr Obes 2011;6 Suppl 1:29–34.
Twisk JW, Kemper HC, Mellenbergh GJ . Mathematical and analytical aspects of tracking. Epidemiol Rev 1994;16:165–83.
Camhi SM, Katzmarzyk PT . Tracking of cardiometabolic risk factor clustering from childhood to adulthood. Int J Pediatr Obes 2010;5:122–9.
Chen W, Srinivasan SR, Elkasabany A, Berenson GS . Cardiovascular risk factors clustering features of insulin resistance syndrome (Syndrome X) in a biracial (Black-White) population of children, adolescents, and young adults: the Bogalusa Heart Study. Am J Epidemiol 1999;150:667–74.
Twisk JW, Kemper HC, van Mechelen W, Post GB . Tracking of risk factors for coronary heart disease over a 14-year period: a comparison between lifestyle and biologic risk factors with data from the Amsterdam Growth and Health Study. Am J Epidemiol 1997;145:888–98.
Rizzo NS, Ruiz JR, Hurtig-Wennlöf A, Ortega FB, Sjöström M . Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: the European youth heart study. J Pediatr 2007;150:388–94.
Andersen LB, Wedderkopp N, Hansen HS, Cooper AR, Froberg K . Biological cardiovascular risk factors cluster in Danish children and adolescents: the European Youth Heart Study. Prev Med 2003;37:363–7.
Artero EG, Ruiz JR, Ortega FB, et al. Muscular and cardiorespiratory fitness are independently associated with metabolic risk in adolescents: the HELENA study. Pediatr Diabetes 2011;12:704–12.
Chen W, Srinivasan SR, Li S, Xu J, Berenson GS . Clustering of long-term trends in metabolic syndrome variables from childhood to adulthood in Blacks and Whites: the Bogalusa Heart Study. Am J Epidemiol 2007;166:527–33.
Martínez-Vizcaino V, Ortega FB, Solera-Martínez M, et al. Stability of the factorial structure of metabolic syndrome from childhood to adolescence: a 6-year follow-up study. Cardiovasc Diabetol 2011;10:81.
Katzmarzyk PT, Pérusse L, Malina RM, Bergeron J, Després JP, Bouchard C . Stability of indicators of the metabolic syndrome from childhood and adolescence to young adulthood: the Québec Family Study. J Clin Epidemiol 2001;54:190–5.
Eisenmann JC, Welk GJ, Wickel EE, Blair SN . Stability of variables associated with the metabolic syndrome from adolescence to adulthood: the Aerobics Center Longitudinal Study. Am J Hum Biol 2004;16:690–6.
Andersen LB, Hasselstrøm H, Grønfeldt V, Hansen SE, Karsten F . The relationship between physical fitness and clustered risk, and tracking of clustered risk from adolescence to young adulthood: eight years follow-up in the Danish Youth and Sport Study. Int J Behav Nutr Phys Act 2004;1:6.
Bao W, Srinivasan SR, Wattigney WA, Berenson GS . Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch Intern Med 1994;154:1842–7.
Hansen SE, Hasselstrom H, Gronfeldt V, Froberg K, Cooper A, Andersen LB . Physical fitness as a predictor of cardiovascular disease risk factors in 6- to 7-year-old Danish children: The Copenhagen School Child Intervention Study. Pediatr Exer Sci 2005;17:55–64.
Juhola J, Magnussen CG, Viikari JS, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr 2011;159:584–90.
Andersen LB, Haraldsdóttir J . Tracking of cardiovascular disease risk factors including maximal oxygen uptake and physical activity from late teenage to adulthood. An 8-year follow-up study. J Intern Med 1993;234:309–15.
McMurray RG, Bangdiwala SI, Harrell JS, Amorim LD . Adolescents with metabolic syndrome have a history of low aerobic fitness and physical activity levels. Dyn Med 2008;7:5.
Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ . Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev 2008;9:474–88.
Garnett SP, Baur LA, Srinivasan S, Lee JW, Cowell CT . Body mass index and waist circumference in midchildhood and adverse cardiovascular disease risk clustering in adolescence. Am J Clin Nutr 2007;86:549–55.
Srinivasan SR, Myers L, Berenson GS . Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes 2002;51:204–9.
Lloyd LJ, Langley-Evans SC, McMullen S . Childhood obesity and adult cardiovascular disease risk: a systematic review. Int J Obes (Lond) 2010;34:18–28.
Bugge A, El-Naaman B, Dencker M, et al. Effects of a three-year intervention: the Copenhagen School Child Intervention Study. Med Sci Sports Exerc 2012;44:1310–7.
Eiberg S, Hasselstrom H, Grønfeldt V, Froberg K, Svensson J, Andersen LB . Maximum oxygen uptake and objectively measured physical activity in Danish children 6-7 years of age: the Copenhagen School Child Intervention Study. Br J Sports Med 2005;39:725–30.
Hasselstrøm HA, Karlsson MK, Hansen SE, Grønfeldt V, Froberg K, Andersen LB . A 3-year physical activity intervention program increases the gain in bone mineral and bone width in prepubertal girls but not boys: the Prospective Copenhagen School Child Interventions Study (CoSCIS). Calcif Tissue Int 2008;83:243–50.
World Health Organization (WHO). (http://www.who.int/childgrowth/en/). Accessed January 2012.
Durnin JV, Rahaman MM . The assessment of the amount of fat in the human body from measurements of skinfold thickness. 1967. Br J Nutr 2003;89:147–55.
McLaughlin JE, King GA, Howley ET, Bassett DR Jr, Ainsworth BE . Validation of the COSMED K4 b2 portable metabolic system. Int J Sports Med 2001;22:280–4.
Jensen K, Jørgensen S, Johansen L . A metabolic cart for measurement of oxygen uptake during human exercise using inspiratory flow rate. Eur J Appl Physiol 2002;87:202–6.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9.
Andersen LB, Harro M, Sardinha LB, et al. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study). Lancet 2006;368:299–304.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bugge, A., El-Naaman, B., McMurray, R. et al. Tracking of clustered cardiovascular disease risk factors from childhood to adolescence. Pediatr Res 73, 245–249 (2013). https://doi.org/10.1038/pr.2012.158
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.158
This article is cited by
-
Fitness, body composition, and metabolic risk scores in children and adolescents: the UP&DOWN study
European Journal of Pediatrics (2022)
-
The stability of cardiometabolic risk factors clustering in children and adolescents: a 2-year longitudinal study
Journal of Diabetes & Metabolic Disorders (2022)
-
Neck circumference and cardiometabolic risk in children and adolescents: the moderator role of cardiorespiratory fitness
BMC Pediatrics (2021)
-
The role of adiposity in the relationship between physical fitness with cardiometabolic risk factors, adipocytokines and inflammation in children
Sport Sciences for Health (2021)
-
The 23-year tracking of blood lipids from adolescence to adulthood in Korea: the Kangwha study
Lipids in Health and Disease (2017)