Abstract
Background:
O-linked β-N-acetylglucosamine (O-GlcNAc) is a glycan essential for fundamental cellular processes such as transcription/translation, nuclear transport, protein stability and protein–protein interactions. However, the role of O-GlcNAc in prostate cancer progression of patients remains poorly unknown. Here we investigated the clinicopathological significance of O-GlcNAc expression level in prostate cancer.
Methods:
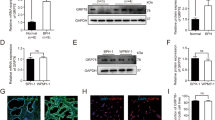
O-GlcNAc expression level in prostate cancer cells was determined by immunohistochemistry of prostate biopsy specimens obtained from 56 patients later treated with hormone deprivation therapy comparing with adjacent normal prostate glands in the same sections. Overall survival was determined by the Kaplan–Meier and Cox proportional hazards methods with univariate and multivariate models. The effects of reduced O-GlcNAc expression level on proliferation and invasion of prostate cancer LNCaP cells were examined using small interfering RNA (siRNA) targeting O-GlcNAc transferase (OGT), the enzyme responsible for O-GlcNAc biosynthesis.
Results:
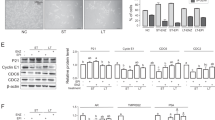
Defining cancer cells showing stronger cytoplasmic staining than normal prostate glands as overexpression of O-GlcNAc, 39% of prostate cancer patients were categorized as overexpression. The Kaplan–Meier and Cox proportional hazards methods with univariate model analysis revealed that O-GlcNAc overexpression was associated with overall survival (P=0.0012 for the Kaplan–Meier and P=0.0021 for Cox univariate hazard model analysis). Furthermore, O-GlcNAc was the only item in which a significant difference was observed at overall survival by multivariate analysis (P=0.0475). Finally, siRNA-mediated OGT knockdown in LNCaP cells resulted in decreased expression of O-GlcNAc and promoted decreased proliferation and tumor cell invasion compared with control siRNA-transfected LNCaP cells.
Conclusions:
These results indicate that O-GlcNAc expression level in prostate cancer cells is associated with poor prognosis of prostate cancer patients and likely enhances tumor cell proliferation and invasion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Torres CR, Hart GW . Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem 1984; 259: 3308–3317.
Slawson C, Housley MP, Hart GW . O-GlcNAc cycling: how a single sugar post-translational modification is changing the way we think about signaling networks. J Cell Biochem 2006; 97: 71–83.
Hart GW, Housley MP, Slawson C . Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007; 446: 1017–1022.
Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X et al. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta 2011; 1812: 514–519.
Yu WG, Gu YC, Mi WY, Ge YQ, Liu HY, Fan QO et al. GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res 2010; 70: 6344–6351.
Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ . Critical role of O-Linked beta-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem 2012; 287: 11070–11081.
Nakayama J, Aoki D, Suga T, Akama TO, Ishizone S, Yamaguchi H et al. Implantation-dependent expression of trophinin by maternal fallopian tube epithelia during tubal pregnancies: possible role of human chorionic gonadotrophin on ectopic pregnancy. Am J Pathol 2003; 163: 2211–2219.
Comer FI, Vosseller K, Wells L, Accavitti MA, Hart GW . Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal Biochem 2001; 293: 169–177.
Harada O, Suga T, Suzuki T, Nakamoto K, Kobayashi M, Nomiyama T et al. The role of trophinin, an adhesion molecule unique to human trophoblasts, in progression of colorectal cancer. Int J Cancer 2007; 121: 1072–1078.
Hart GW, Slawson C . O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer 2011; 11: 678–684.
Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene 2010; 29: 2831–2842.
DeBerardinis RJ . Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med 2008; 10: 767–777.
Wang RY, Gallager DW, Aghajanian GK . Stimulation of pontine reticular formation suppresses firing of serotonergic neuronses in the dorsal raphe. Nature 1976; 264: 365–368.
Vander Heiden MG, Cantley LC, Thompson CB . Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033.
Slawson C, Copeland RJ, Hart GW . O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci 2010; 35: 547–555.
Eagle H, Oyama VI, Levy M, Horton CL, Fleischman R . The growth response of mammalian cells in tissue culture to L-glutamine and L-glutamic acid. J Biol Chem 1956; 218: 607–616.
Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB . Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev 2008; 18: 54–61.
McClain DA, Crook ED . Hexosamines and insulin resistance. Diabetes 1996; 45: 1003–1009.
Acknowledgements
We thank Dr Elise Lamar for editing of the manuscript. This work was supported in part by a Grant-in-Aid for Scientific Research 24390086 from the Japan Society for the Promotion of Science (to JN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kamigaito, T., Okaneya, T., Kawakubo, M. et al. Overexpression of O-GlcNAc by prostate cancer cells is significantly associated with poor prognosis of patients. Prostate Cancer Prostatic Dis 17, 18–22 (2014). https://doi.org/10.1038/pcan.2013.56
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2013.56
Keywords
This article is cited by
-
Salivary metabolomics in oral potentially malignant disorders and oral cancer patients—a systematic review with meta-analysis
Clinical Oral Investigations (2024)
-
O-GlcNAcylation: an important post-translational modification and a potential therapeutic target for cancer therapy
Molecular Medicine (2022)
-
O-GlcNAcylation links oncogenic signals and cancer epigenetics
Discover Oncology (2021)
-
Potential coordination role between O-GlcNAcylation and epigenetics
Protein & Cell (2017)
-
The role of glycans in the development and progression of prostate cancer
Nature Reviews Urology (2016)