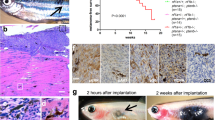
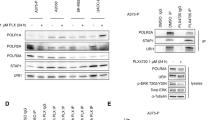
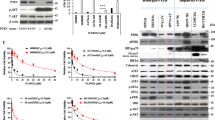
Abstract
Activating mutations in the RAC1 gene have recently been discovered as driver events in malignant melanoma. Expression of this gene is associated with melanocyte proliferation, and melanoma cells bearing this mutation are insensitive to BRAF inhibitors such as vemurafenib and dabrafenib, and also may evade immune surveillance due to enhanced expression of PD-L1. Activating mutations in RAC1 are of special interest, as small-molecule inhibitors for the RAC effector p21-activated kinase (PAK) are in late-stage clinical development and might impede oncogenic signaling from mutant RAC1. In this work, we explore the effects of PAK inhibition on RAC1P29S signaling in zebrafish embryonic development, in the proliferation, survival and motility of RAC1P29S-mutant human melanoma cells, and on tumor formation and progression from such cells in mice. We report that RAC1P29S evokes a Rasopathy-like phenotype on zebrafish development that can be blocked by inhibitors of PAK or MEK. We also found and that RAC1-mutant human melanoma cells are resistant to clinical inhibitors of BRAF but are uniquely sensitive to PAK inhibitors. These data suggest that suppressing the PAK pathway might be of therapeutic benefit in this type of melanoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Davis MJ, Ha BH, Holman EC, Halaban R, Schlessinger J, Boggon TJ . RAC1P29S is a spontaneously activating cancer-associated GTPase. Proc Natl Acad Sci USA 2013; 110: 912–917.
Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP et al. A landscape of driver mutations in melanoma. Cell 2012; 150: 251–263.
Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 2012; 44: 1006–1014.
Watson IR, Li L, Cabeceiras PK, Mahdavi M, Gutschner T, Genovese G et al. The RAC1 P29S hotspot mutation in melanoma confers resistance to pharmacological inhibition of RAF. Cancer Res 2014; 74: 4845–4852.
Vu HL, Rosenbaum S, Purwin TJ, Davies MA, Aplin AE . RAC1 P29S regulates PD-L1 expression in melanoma. Pigment Cell Melanoma Res 2015; 28: 590–598.
Kawazu M, Ueno T, Kontani K, Ogita Y, Ando M, Fukumura K et al. Transforming mutations of RAC guanosine triphosphatases in human cancers. Proc Natl Acad Sci USA 2013; 110: 3029–3034.
Qiu R-G, Chen J, Kirn D, McCormick F, Symons M . An essential role for Rac in Ras transformation. Nature 1995; 374: 457–459.
Radu M, Semenova G, Kosoff R, Chernoff J . PAK signalling during the development and progression of cancer. Nat Rev Cancer 2014; 14: 13–25.
Ong CC, Jubb AM, Jakubiak D, Zhou W, Rudolph J, Haverty PM et al. P21-activated kinase 1 (PAK1) as a therapeutic target in BRAF wild-type melanoma. J Natl Cancer Inst 2013; 105: 606–607.
Chow HY, Jubb AM, Koch JN, Jaffer ZM, Stepanova D, Campbell DA et al. p21-activated kinase 1 is required for efficient tumor formation and progression in a Ras-mediated skin cancer model. Cancer Res 2012; 72: 5966–5975.
Anastasaki C, Estep AL, Marais R, Rauen KA, Patton EE . Kinase-activating and kinase-impaired cardio-facio-cutaneous syndrome alleles have activity during zebrafish development and are sensitive to small molecule inhibitors. Hum Mol Genet 2009; 18: 2543–2554.
Halaban R, Krauthammer M . RASopathy gene mutations in melanoma. J Invest Dermatol 2016; 136: 1755–1759.
Jindal GA, Goyal Y, Burdine RD, Rauen KA, Shvartsman SY . RASopathies: unraveling mechanisms with animal models. Dis Model Mech 2015; 8: 769–782.
Anastasaki C, Rauen KA, Patton EE . Continual low-level MEK inhibition ameliorates cardio-facio-cutaneous phenotypes in zebrafish. Dis Model Mech 2012; 5: 546–552.
Ong CC, Gierke S, Pitt C, Sagolla M, Cheng CK, Zhou W et al. Small molecule inhibition of group I p21-activated kinases in breast cancer induces apoptosis and potentiates the activity of microtubule stabilizing agents. Breast Cancer Res 2015; 17: 59.
Semenova G, Chernoff J . Targeting PAK1. Biochem Soc Trans 2017; 45: 79–88.
Grzmil M, Whiting D, Maule J, Anastasaki C, Amatruda JF, Kelsh RN et al. The INT6 cancer gene and MEK signaling pathways converge during zebrafish development. PLoS One 2007; 2: e959.
Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y . Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 2004; 101: 7618–7623.
Halaban R . RAC1 and melanoma. Clin Ther 2015; 37: 682–685.
Krauthammer M, Kong Y, Bacchiocchi A, Evans P, Pornputtapong N, Wu C et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat Genet 2015; 47: 996–1002.
Li A, Ma Y, Yu X, Mort RL, Lindsay CR, Stevenson D et al. Rac1 drives melanoblast organization during mouse development by orchestrating pseudopod-driven motility and cell-cycle progression. Dev Cell 2011; 21: 722–734.
Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS et al. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell 2013; 153: 1050–1063.
Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 2014; 25: 831–845.
Lindsay CR, Lawn S, Campbell AD, Faller WJ, Rambow F, Mort RL et al. P-Rex1 is required for efficient melanoblast migration and melanoma metastasis. Nat Commun 2011; 2: 555.
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF . Stages of embryonic development of the zebrafish. Dev Dyn 1995; 203: 253–310.
Shin JT, Priest JR, Ovcharenko I, Ronco A, Moore RK, Burns CG et al. Human-zebrafish non-coding conserved elements act in vivo to regulate transcription. Nucleic Acids Res 2005; 33: 5437–5445.
Rhodes J, Amsterdam A, Sanda T, Moreau LA, McKenna K, Heinrichs S et al. Emi1 maintains genomic integrity during zebrafish embryogenesis and cooperates with p53 in tumor suppression. Mol Cell Biol 2009; 29: 5911–5922.
Lightcap CM, Kari G, Arias-Romero LE, Chernoff J, Rodeck U, Williams JC . Interaction with LC8 is required for Pak1 nuclear import and is indispensable for zebrafish development. PLoS One 2009; 4: e6025.
Link V, Shevchenko A, Heisenberg CP . Proteomics of early zebrafish embryos. BMC Dev Biol 2006; 6: 1.
Limame R, Wouters A, Pauwels B, Fransen E, Peeters M, Lardon F et al. Comparative analysis of dynamic cell viability, migration and invasion assessments by novel real-time technology and classic endpoint assays. PLoS One 2012; 7: e46536.
Acknowledgements
We thank Drs Ruth Halaban and Meenhard Herlyn for providing melanoma cell lines, and Dr Rebecca Burdine for Tg(cmlc2:EGFP) zebrafish, Genentech for providing Frax-1036 and the Fox Chase Cancer Center Animal Facility for assistance with zebrafish experiments. This work was supported by R01CA227184 (JC), NIH CORE Grant P30 CA006927, and an appropriation from the state of Pennsylvania to the Fox Chase Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Araiza-Olivera, D., Feng, Y., Semenova, G. et al. Suppression of RAC1-driven malignant melanoma by group A PAK inhibitors. Oncogene 37, 944–952 (2018). https://doi.org/10.1038/onc.2017.400
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.400
This article is cited by
-
A comprehensive signature based on endoplasmic reticulum stress-related genes in predicting prognosis and immunotherapy response in melanoma
Scientific Reports (2023)
-
P21-activated kinase 2-mediated β-catenin signaling promotes cancer stemness and osimertinib resistance in EGFR-mutant non-small-cell lung cancer
Oncogene (2022)
-
Coordinated dysregulation of cancer progression by the HER family and p21-activated kinases
Cancer and Metastasis Reviews (2020)
-
Targeting p21-activated kinase 1 inhibits growth and metastasis via Raf1/MEK1/ERK signaling in esophageal squamous cell carcinoma cells
Cell Communication and Signaling (2019)
-
Rho GTPases in cancer: friend or foe?
Oncogene (2019)