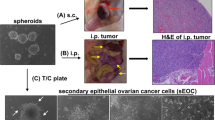
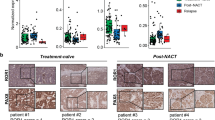
Abstract
Mechanisms underlying ovarian cancer initiation and progression are unclear. Herein, we report that the Yes-associated protein (YAP), a major effector of the Hippo tumor suppressor pathway, interacts with ERBB signaling pathways to regulate the initiation and progression of ovarian cancer. Immunohistochemistry studies indicate that YAP expression is associated with poor clinical outcomes in patients. Overexpression or constitutive activation of YAP leads to transformation and tumorigenesis in human ovarian surface epithelial cells, and promotes growth of cancer cells in vivo and in vitro. YAP induces the expression of epidermal growth factor (EGF) receptors (EGFR, ERBB3) and production of EGF-like ligands (HBEGF, NRG1 and NRG2). HBEGF or NRG1, in turn, activates YAP and stimulates cancer cell growth. Knockdown of ERBB3 or HBEGF eliminates YAP effects on cell growth and transformation, whereas knockdown of YAP abrogates NRG1- and HBEGF-stimulated cell proliferation. Collectively, our study demonstrates the existence of HBEGF & NRGs/ERBBs/YAP/HBEGF & NRGs autocrine loop that controls ovarian cell tumorigenesis and cancer progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Siegel R, Desantis C, Jemal A . Colorectal cancer statistics 2014 CA Cancer J Clin 2014; 64: 104–117.
Bast Jr RC, Hennessy B, Mills GB . The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 2009; 9: 415–428.
Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ . The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev 1995; 9: 534–546.
Xu T, Wang W, Zhang S, Stewart RA, Yu W . Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 1995; 121: 1053–1063.
Pan D . The hippo signaling pathway in development and cancer. Dev Cell 2010; 19: 491–505.
Yu FX, Guan KL . The Hippo pathway: regulators and regulations. Genes Dev 2013; 27: 355–371.
Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007; 130: 1120–1133.
Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA 2010; 107: 8248–8253.
Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res 2010; 70: 8517–8525.
Zhang X, George J, Deb S, Degoutin JL, Takano EA, Fox SB et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 2011; 30: 2810–2822.
Harvey KF, Zhang X, Thomas DM . The Hippo pathway and human cancer. Nat Rev Cancer 2013; 13: 246–257.
Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ 2008; 15: 1752–1759.
Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA damage. Mol Cell 2005; 18: 447–459.
Cai H, Xu Y . The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun Signal 2013; 11: 31.
Lucas EP, Khanal I, Gaspar P, Fletcher GC, Polesello C, Tapon N et al. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J Cell Biol 2013; 201: 875–885.
Kojic N, Chung E, Kho AT, Park JA, Huang A, So PT et al. An EGFR autocrine loop encodes a slow-reacting but dominant mode of mechanotransduction in a polarized epithelium. FASEB J 2010; 24: 1604–1615.
Tschumperlin DJ . EGFR autocrine signaling in a compliant interstitial space: mechanotransduction from the outside. in. Cell Cycle 2004; 3: 996–997.
Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI . Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol 2007; 177: 893–903.
Fan R, Kim NG, Gumbiner BM . Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A 2013; 110: 2569–2574.
Reddy BV, Irvine KD . Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell 2013; 24: 459–471.
Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012; 150: 780–791.
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007; 21: 2747–2761.
Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev 2012; 26: 1300–1305.
Yang N, Morrison CD, Liu P, Miecznikowski J, Bshara W, Han S et al. TAZ induces growth factor-independent proliferation through activation of EGFR ligand amphiregulin. Cell Cycle 2012; 11: 2922–2930.
Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol 2009; 11: 1444–1450.
Hynes NE, Lane HA . ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005; 5: 341–354.
Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol 2008; 39: 1582–1589.
Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X . Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci 2010; 101: 1279–1285.
Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer 2009; 115: 4576–4585.
Xia Y, Chang T, Wang Y, Liu Y, Li W, Li M et al. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PloS One 2014; 9: e91770.
Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO . The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A 2012; 109: E2441–E2450.
Kim JM, Kang DW, Long LZ, Huang SM, Yeo MK, Yi ES et al. Differential expression of Yes-associated protein is correlated with expression of cell cycle markers and pathologic TNM staging in non-small-cell lung carcinoma. Hum Pathol 2011; 42: 315–323.
Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC . Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 2001; 22: 255–288.
Orsulic S, Li Y, Soslow RA, Vitale-Cross LA, Gutkind JS, Varmus HE . Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell 2002; 1: 53–62.
Gregoire L, Rabah R, Schmelz EM, Munkarah A, Roberts PC, Lancaster WD . Spontaneous malignant transformation of human ovarian surface epithelial cells in vitro. Clin Cancer Res 2001; 7: 4280–4287.
Liu J, Yang G, Thompson-Lanza JA, Glassman A, Hayes K, Patterson A et al. A genetically defined model for human ovarian cancer. Cancer Res 2004; 64: 1655–1663.
Sasaki R, Narisawa-Saito M, Yugawa T, Fujita M, Tashiro H, Katabuchi H et al. Oncogenic transformation of human ovarian surface epithelial cells with defined cellular oncogenes. Carcinogenesis 2009; 30: 423–431.
Omerovic J, Puggioni EM, Napoletano S, Visco V, Fraioli R, Frati L et al. Ligand-regulated association of ErbB-4 to the transcriptional co-activator YAP65 controls transcription at the nuclear level. Exp Cell Res 2004; 294: 469–479.
Komuro A, Nagai M, Navin NE, Sudol M . WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem 2003; 278: 33334–33341.
Sheng Q, Liu X, Fleming E, Yuan K, Piao H, Chen J et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell 2010; 17: 298–310.
Miyamoto S, Hirata M, Yamazaki A, Kageyama T, Hasuwa H, Mizushima H et al. Heparin-binding EGF-like growth factor is a promising target for ovarian cancer therapy. Cancer Res 2004; 64: 5720–5727.
Roepstorff K, Grandal MV, Henriksen L, Knudsen SL, Lerdrup M, Grovdal L et al. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic 2009; 10: 1115–1127.
Banerjee S, Kaye SB . New strategies in the treatment of ovarian cancer: current clinical perspectives and future potential. Clin Cancer Res 2013; 19: 961–968.
Wang C, Roy SK . Expression of E-cadherin and N-cadherin in perinatal hamster ovary: possible involvement in primordial follicle formation and regulation by follicle-stimulating hormone. Endocrinology 2010; 151: 2319–2330.
Fu D, Lv X, Hua G, He C, Dong J, Lele SM et al. YAP regulates cell proliferation, migration, and steroidogenesis in adult granulosa cell tumors. Endocr Relat Cancer 2014; 21: 297–310.
Amsellem-Ouazana D, Bieche I, Tozlu S, Botto H, Debre B, Lidereau R . Gene expression profiling of ERBB receptors and ligands in human transitional cell carcinoma of the bladder. J Urology 2006; 175: 1127–1132.
Acknowledgements
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5R00HD059985, 5P01AG029531); The Olson Center for Women’s Health (no number); The Fred & Pamela Buffett Cancer Center (LB595); the Omaha Veterans Administration Medical Center (5I01BX000512) and The National Institute of Food and Agriculture (2011-67015-20076). We wish to thank for Melody A. Montgomery at the University of Nebraska Medical Center Research Editorial Office for her professional assistance in editing this manuscript.
Author Contributions
CH contributed to the design, performance, data analysis and manuscript preparation. XL packaged the retrovirus and performed in vivo experiments. GH conducted IHC analysis. JD constructed the retroviral vectors. SL and SR evaluated and analyzed patient samples. JSD contributed to the experimental design, results, discussion and manuscript preparation. CW supervised these studies and contributed to the experimental design, data analysis and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
He, C., Lv, X., Hua, G. et al. YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression. Oncogene 34, 6040–6054 (2015). https://doi.org/10.1038/onc.2015.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.52
This article is cited by
-
Insights into recent findings and clinical application of YAP and TAZ in cancer
Nature Reviews Cancer (2023)
-
Human epidermal growth factor receptor 3 serves as a novel therapeutic target for acral melanoma
Cell Death Discovery (2023)
-
HLF promotes ovarian cancer progression and chemoresistance via regulating Hippo signaling pathway
Cell Death & Disease (2023)
-
The diverse functions of FAT1 in cancer progression: good, bad, or ugly?
Journal of Experimental & Clinical Cancer Research (2022)
-
Yap haploinsufficiency leads to Müller cell dysfunction and late-onset cone dystrophy
Cell Death & Disease (2020)