Abstract
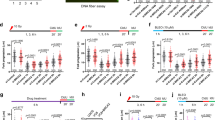
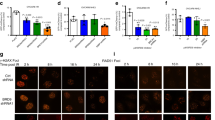
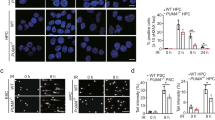
The BMI1 protein contributes to stem cell pluripotency and oncogenesis via multiple functions, including its newly identified role in DNA damage response (DDR). Although evidence clearly demonstrates that BMI1 facilitates the repair of double-stranded breaks via homologous recombination (HR), it remains unclear how BMI1 regulates checkpoint activation during DDR. We report here that BMI1 has a role in G2/M checkpoint activation in response to etoposide (ETOP) treatment. Ectopic expression of BMI1 in MCF7 breast cancer and DU145 prostate cancer cells significantly reduced ETOP-induced G2/M arrest. Conversely, knockdown of BMI1 in both lines enhanced the arrest. Consistent with ETOP-induced activation of the G2/M checkpoints via the ATM pathway, overexpression and knockdown of BMI1, respectively, reduced and enhanced ETOP-induced phosphorylation of ATM at serine 1981 (ATM pS1981). Furthermore, the phosphorylation of ATM targets, including γH2AX, threonine 68 (T68) on CHK2 (CHK2 pT68) and serine 15 (S15) on p53 were decreased in overexpression and increased in knockdown BMI1 cells in response to ETOP. In line with the requirement of NBS1 in ATM activation, we were able to show that BMI1 associates with NBS1 and that this interaction altered the binding of NBS1 with ATM. BMI1 consists of a ring finger (RF), helix-turn-helix-turn-helix-turn (HT), proline/serine (PS) domain and two nuclear localization signals (NLS). Although deletion of either RF or HT did not affect the association of BMI1 with NBS1, the individual deletions of PS and one NLS (KRMK) robustly reduced the interaction. Stable expression of these BMI1 mutants decreased ETOP-induced ATM pS1981 and CHK2 pT68, but not ETOP-elicited γH2AX in MCF7 cells. Furthermore, ectopic expression of BMI1 in non-transformed breast epithelial MCF10A cells also compromised ETOP-initiated ATM pS1981 and γH2AX. Taken together, we provide compelling evidence that BMI1 decreases ETOP-induced G2/M checkpoint activation via reducing NBS1-mediated ATM activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 2003; 423: 302–305.
Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ . Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 2003; 425: 962–967.
Bruggeman SW, Valk-Lingbeek ME, van der Stoop PP, Jacobs JJ, Kieboom K, Tanger E et al. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev 2005; 19: 1438–1443.
Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R . Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev 2005; 19: 1432–1437.
Chagraoui J, Niessen SL, Lessard J, Girard S, Coulombe P, Sauvageau M et al. E4F1: a novel candidate factor for mediating BMI1 function in primitive hematopoietic cells. Genes Dev 2006; 20: 2110–2120.
Akala OO, Park IK, Qian D, Pihalja M, Becker MW, Clarke MF . Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature 2008; 453: 228–232.
Quelle DE, Zindy F, Ashmun RA, Sherr CJ . Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 1995; 83: 993–1000.
Sherr CJ . Tumor surveillance via the ARF-p53 pathway. Genes Dev 1998; 12: 2984–2991.
Haupt Y, Alexander WS, Barri G, Klinken SP, Adams JM . Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell 1991; 65: 753–763.
van Lohuizen M, Verbeek S, Scheijen B, Wientjens E, van der Gulden H, Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell 1991; 65: 737–752.
Haupt Y, Bath ML, Harris AW, Adams JM . bmi-1 transgene induces lymphomas and collaborates with myc in tumorigenesis. Oncogene 1993; 8: 3161–3164.
Alkema MJ, Jacobs H, van Lohuizen M, Berns A . Pertubation of B and T cell development and predisposition to lymphomagenesis in Emu Bmi1 transgenic mice require the Bmi1 RING finger. Oncogene 1997; 15: 899–8910.
Bea S, Tort F, Pinyol M, Puig X, Hernández L, Hernández S et al. BMI-1 gene amplification and overexpression in hematological malignancies occur mainly in mantle cell lymphomas. Cancer Res 2001; 61: 2409–2412.
van Galen JC, Muris JJ, Oudejans JJ, Vos W, Giroth CP, Ossenkoppele GJ et al. Expression of the polycomb-group gene BMI1 is related to an unfavourable prognosis in primary nodal DLBCL. J Clin Pathol 2007; 60: 167–172.
Mihara K, Chowdhury M, Nakaju N, Hidani S, Ihara A, Hyodo H et al. Bmi-1 is useful as a novel molecular marker for predicting progression of myelodysplastic syndrome and patient prognosis. Blood 2006; 107: 305–308.
Vonlanthen S, Heighway J, Altermatt HJ, Gugger M, Kappeler A, Borner MM et al. The bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A-ARF locus expression. Br J Cancer 2001; 84: 1372–1376.
Kim JH, Yoon SY, Kim CN, Joo JH, Moon SK, Choe IS et al. The Bmi-1 oncoprotein is overexpressed in human colorectal cancer and correlates with the reduced p16INK4a/p14ARF proteins. Cancer Lett 2004; 203: 217–224.
Kim JH, Yoon SY, Jeong SH, Kim SY, Moon SK, Joo JH et al. Overexpression of Bmi-1 oncoprotein correlates with axillary lymph node metastases in invasive ductal breast cancer. Breast 2004; 13: 383–388.
Song LB, Zeng MS, Liao WT, Zhang L, Mo HY, Liu WL et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res 2006; 66: 6225–6232.
Levine SS, King IF, Kingston RE . Division of labor in polycomb group repression. Trends Biochem Sci 2004; 29: 478–485.
Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature 2004; 431: 873–878.
deNapoles M, Mermoud JE, Wakao R, Tang YA, Endoh M, Appanah R et al. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell 2004; 7: 663–676.
Cao R, Tsukada Y, Zhang Y . Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell 2005; 20: 845–854.
Li Z, Cao R, Wang M, Myers MP, Zhang Y, Xu RM . Structure of a Bmi-1-Ring1B polycomb group ubiquitin ligase complex. J Biol Chem 2006; 281: 20643–20649.
Zhou BB, Elledge SJ . The DNA damage response: putting checkpoints in perspective. Nature 2000; 408: 433–439.
Lengauer C, Kinzler KW, Vogelstein B . Genetic instabilities in human cancers. Nature 1998; 396: 643–649.
Hoeijmakers JH . Genome maintenance mechanisms for preventing cancer. Nature 2001; 411: 366–374.
Rouse J, Jackson SP . Interfaces between the detection, signaling, and repair of DNA damage. Science 2002; 297: 547–551.
Shiloh Y . ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 2003; 3: 155–168.
Ismail IH, Andrin C, McDonald D, Hendzel MJ . BMI1-mediated histone ubiquitylation promotes DNA double-strand break repair. J Cell Biol 2010; 191: 45–60.
Chagraoui J, Hébert J, Girard S, Sauvageau G . An anticlastogenic function for the Polycomb Group gene Bmi1. Proc Natl Acad Sci USA 2011; 108: 5284–5289.
Ginjala V, Nacerddine K, Kulkarni A, Oza J, Hill SJ, Yao M et al. BMI1 is recruited to DNA breaks and contributes to DNA damage-induced H2A ubiquitination and repair. Mol Cell Biol 2011; 31: 1972–1982.
Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM . A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 2000; 10: 886–895.
Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA et al. Genomic instability in mice lacking histone H2AX. Science 2002; 296: 922–927.
Crea F, Duhagon Serrat MA, Hurt EM, Thomas SB, Danesi R, Farrar WL . BMI1 silencing enhances docetaxel activity and impairs antioxidant response in prostate cancer. Int J Cancer 2011; 128: 1946–1954.
Facchino S, Abdouh M, Chatoo W, Bernier G . BMI1 confers radioresistance to normal and cancerous neural stem cells through recruitment of the DNA damage response machinery. J Neurosci 2010; 30: 10096–10111.
Gieni RS, Ismail IH, Campbell S, Hendzel MJ . Polycomb group proteins in the DNA damage response: a link between radiation resistance and ‘stemness’. Cell Cycle 2011; 10: 883–894.
Liu ZG, Liu L, Xu LH, Yi W, Tao YL, Tu ZW et al. Bmi-1 induces radioresistance in MCF-7 mammary carcinoma cells. Oncol Rep 2012; 27: 1116–1122.
Sieber OM, Heinimann K, Tomlinson IP . Genomic instability—the engine of tumorigenesis? Nat Rev Cancer 2003; 3: 701–708.
Kops GJ, Weaver BA, Cleveland DW . On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer 2005; 5: 773–785.
O'Driscoll M, Jeggo PA . The role of double-strand break repair—insights from human genetics. Nat Rev Genet 2006; 7: 45–54.
Wei F, Xie Y, Tao L, Tang D . Both ERK1 and ERK2 kinases promote G2/M arrest in etoposide-treated MCF7 cells by facilitating ATM activation. Cell Signal 2010; 22: 1783–1789.
Bakkenist CJ, Kastan MB . DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003; 421: 499–506.
Dasika GK, Lin SC, Zhao S, Sung P, Tomkinson A, Lee EY . DNA damage-induced cell cycle checkpoints and DNA strand break repair in development and tumorigenesis. Oncogene 1999; 18: 7883–7899.
Kurz EU, Lees-Miller SP . DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair 2004; 3: 889–900.
Abraham RT . Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 2001; 15: 2177–2196.
Lavin MF, Gueven N . The complexity of p53 stabilization and activation. Cell Death Differ 2006; 13: 941–950.
Goto H, Tomono Y, Ajiro K, Kosako H, Fujita M, Sakurai M et al. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem 1999; 274: 25543–25549.
Prigent C, Dimitrov S . Phosphorylation of serine 10 in histone H3, what for? J Cell Sci 2003; 116: 3677–3685.
Difilippantonio S, Celeste A, Fernandez-Capetillo O, Chen HT, Reina San Martin B, Van Laethem F et al. Role of Nbs1 in the activation of the Atm kinase revealed in humanized mouse models. Nat Cell Biol 2005; 7: 675–685.
Falck J, Coates J, Jackson SP . Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 2005; 434: 605–661.
Cohen KJ, Hanna JS, Prescott JE, Dang CV . Transformation by the Bmi-1 oncoprotein correlates with its subnuclear localization but not its transcriptional suppression activity. Mol Cell Biol 1996; 16: 5527–5535.
Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006; 444: 633–637.
Halazonetis TD, Gorgoulis VG, Bartek J . An oncogene-induced DNA damage model for cancer development. Science 2008; 319: 1352–1355.
Glinsky GV, Berezovska O, Glinskii AB . Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest 2005; 115: 1503–1521.
Nacerddine K, Beaudry JB, Ginjala V, Westerman B, Mattiroli F, Song JY et al. Akt-mediated phosphorylation of Bmi1 modulates its oncogenic potential, E3 ligase activity, and DNA damage repair activity in mouse prostate cancer. J Clin Invest 2012; 122: 1920–1932.
Siddique HR, Saleem M . Role of BMI1, a stem cell factor, in cancer recurrence and chemoresistance: preclinical and clinical evidences. Stem Cells 2012; 30: 372–378.
Pan MR, Peng G, Hung WC, Lin SY . Monoubiquitination of H2AX protein regulates DNA damage response signaling. J Biol Chem 2011; 286: 28599–28607.
Dong Q, Oh JE, Chen W, Kim R, Kim RH, Shin KH et al. Radioprotective effects of Bmi-1 involve epigenetic silencing of oxidase genes and enhanced DNA repair in normal human keratinocytes. J Invest Dermatol 2011; 131: 1216–1225.
Wang E, Bhattacharyya S, Szabolcs A, Rodriguez-Aguayo C, Jennings NB, Lopez-Berestein G et al. Enhancing chemotherapy response with Bmi-1 silencing in ovarian cancer. PLoS ONE 2011; 6: e17918.
Gatei M, Young D, Cerosaletti KM, Desai-Mehta A, Spring K, Kozlov S et al. ATM-dependent phosphorylation of nibrin in response to radiation exposure. Nat Genet 2000; 25: 115–119.
Hemenway CS, Halligan BW, Levy LS . The Bmi-1 oncoprotein interacts with dinG and MPh2: the role of RING finger domains. Oncogene 1998; 16: 2541–2547.
Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B et al. Erk activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem 2002; 277: 12710–12717.
Tang D, Okada H, Ruland J, Liu L, Stambolic V, Mak TW et al. Akt is activated in response to an apoptotic signal. J Biol Chem 2001; 276: 30461–30466.
Acknowledgements
This work was supported by a CIHR grant (MOP-84381) to D Tang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Wei, F., Ojo, D., Lin, X. et al. BMI1 attenuates etoposide-induced G2/M checkpoints via reducing ATM activation. Oncogene 34, 3063–3075 (2015). https://doi.org/10.1038/onc.2014.235
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.235
This article is cited by
-
Redox-dependent BMI1 activity drives in vivo adult cardiac progenitor cell differentiation
Cell Death & Differentiation (2018)
-
The novel BMI-1 inhibitor PTC596 downregulates MCL-1 and induces p53-independent mitochondrial apoptosis in acute myeloid leukemia progenitor cells
Blood Cancer Journal (2017)
-
miR-15a/miR-16 down-regulates BMI1, impacting Ub-H2A mediated DNA repair and breast cancer cell sensitivity to doxorubicin
Scientific Reports (2017)