Abstract
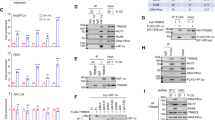
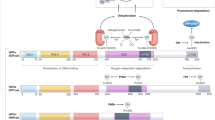
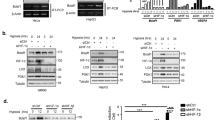
Hypoxia-inducible factors (HIFs) mediate the transcriptional adaptation of hypoxic cells. The extensive transcriptional programm regulated by HIFs involves the induction of genes controlling angiogenesis, cellular metabolism, cell growth, metastasis, apoptosis, extracellular matrix remodeling and others. HIF is a heterodimer of HIF-α and HIF-β subunits. In addition to HIF-1α, HIF-2α has evolved as an isoform that contributes differently to the hypoxic adaptation by performing non-redundant functions. Poly (ADP-ribose) polymerase-1 (PARP-1) is a nuclear protein involved in the control of DNA repair and gene transcription by modulating chromatin structure and acting as part of gene-specific enhancer/promoter-binding complexes. Previous results have shown that PARP-1 regulates HIF-1 activity. In this study, we focused on the cross-talk between HIF-2α and PARP-1. By using different approaches to suppress PARP-1, we show that HIF-2α mRNA expression, protein levels and HIF-2-dependent gene expression, such as ANGPTL4 and erythropoietin (EPO), are regulated by PARP-1. This regulation occurs at both the transcriptional and post-trancriptional level. We also show a complex formation between HIF-2α with PARP-1. This complex is sensitive to PARP inhibition and seems to protect against the von Hippel–Lindau-dependent HIF-2α degradation. Finally, we show that parp-1−/− mice display a significant reduction in the circulating hypoxia-induced EPO levels, number of red cells and hemoglobin concentration. Altogether, these results reveal a complex functional interaction between PARP-1 and the HIF system and suggest that PARP-1 is involved in the fine tuning of the HIF-mediated hypoxic response in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Semenza GL . Hypoxia-inducible factors in physiology and medicine. Cell 2012; 148: 399–408.
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998; 394: 485–490.
Hochachka PW, Buck LT, Doll CJ, Land SC . Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA 1996; 93: 9493–9498.
Benizri E, Ginouves A, Berra E . The magic of the hypoxia-signaling cascade. Cell Mol Life Sci 2008; 65: 1133–1149.
Wang GL, Jiang BH, Rue EA, Semenza GL . Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995; 92: 5510–5514.
Pugh CW, Tan CC, Jones RW, Ratcliffe PJ . Functional analysis of an oxygen-regulated transcriptional enhancer lying 3′ to the mouse erythropoietin gene. Proc Natl Acad Sci USA 1991; 88: 10553–10557.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999; 399: 271–275.
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001; 292: 464–468.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001; 292: 468–472.
Bruick RK, McKnight SL . A conserved family of prolyl-4-hydroxylases that modify HIF. Science 2001; 294: 1337–1340.
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001; 107: 43–54.
Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK . FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 2002; 16: 1466–1471.
Tian H, McKnight SL, Russell DW . Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 1997; 11: 72–82.
Ryan HE, Lo J, Johnson RS . HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 1998; 17: 3005–3015.
Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 1998; 12: 149–162.
Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL . The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev 1998; 12: 3320–3324.
Peng J, Zhang L, Drysdale L, Fong GH . The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl Acad Sci USA 2000; 97: 8386–8391.
Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev 2006; 20: 557–570.
Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR . High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 2011; 117: e207–e217.
Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 2007; 117: 1068–1077.
Aprelikova O, Wood M, Tackett S, Chandramouli GV, Barrett JC . Role of ETS transcription factors in the hypoxia-inducible factor-2 target gene selection. Cancer Res 2006; 66: 5641–5647.
Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE . HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J 2004; 23: 1949–1956.
Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC . HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 2007; 11: 335–347.
Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C . HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest 2009; 119: 1159–1166.
Loboda A, Jozkowicz A, Dulak J . HIF-1 versus HIF-2—is one more important than the other? Vascul Pharmacol 2012; 56: 245–251.
Schreiber V, Dantzer F, Ame JC, de Murcia G . Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 2006; 7: 517–528.
Aguilar-Quesada R, Munoz-Gamez JA, Martin-Oliva D, Peralta-Leal A, Quiles-Perez R, Rodriguez-Vargas JM et al. Modulation of transcription by PARP-1: consequences in carcinogenesis and inflammation. Curr Med Chem 2007; 14: 1179–1187.
Kraus WL, Lis JT . PARP goes transcription. Cell 2003; 113: 677–683.
Martinez-Romero R, Canuelo A, Siles E, Oliver FJ, Martinez-Lara E . Nitric oxide modulates hypoxia-inducible factor-1 and poly(ADP-ribose) polymerase-1 cross-talk in response to hypobaric hypoxia. J Appl Physiol 2012; 112: 816–823.
Elser M, Borsig L, Hassa PO, Erener S, Messner S, Valovka T et al. Poly(ADP-ribose) polymerase 1 promotes tumor cell survival by coactivating hypoxia-inducible factor-1-dependent gene expression. Mol Cancer Res 2008; 6: 282–290.
Yoon D, Ponka P, Prchal JT . Hypoxia and hematopoiesis. Am J Physiol 2011; 300: C1215–C1222.
Rosenberger C, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U et al. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 2002; 13: 1721–1732.
Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD . The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell 2002; 1: 247–255.
Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol 2005; 25: 5675–5686.
Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG . Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell 2002; 1: 237–246.
Kondo K, Kim WY, Lechpammer M, Kaelin WG . Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol 2003; 1: E83.
Flamme I, Krieg M, Plate KH . Up-regulation of vascular endothelial growth factor in stromal cells of hemangioblastomas is correlated with up-regulation of the transcription factor HRF/HIF-2alpha. Am J Pathol 1998; 153: 25–29.
Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 2006; 10: 413–423.
de Murcia JM, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M et al. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci USA 1997; 94: 7303–7307.
Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J . HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003; 22: 4082–4090.
Pescador N, Cuevas Y, Naranjo S, Alcaide M, Villar D, Landazuri MO et al. Identification of a functional hypoxia-responsive element that regulates the expression of the egl nine homologue 3 (egln3/phd3) gene. Biochem J 2005; 390 (Pt 1): 189–197.
Acknowledgements
We thank Dr Françoise Dantzer, CNRS, Strasbourg (France), for providing the pEGFP-PARP-1 construct. This work was supported by Ministerio de Ciencia e Innovación (SAF2006-01094 and SAF2009-13281-C02-01), Fundación La Caixa (BM06-219-0) and Junta de Andalucía (P07-CTS-0239) to FJO; Ministerio de Educación y Ciencia (SAF2007-64597 and SAF-2010-20067) and the BIZKAIA XEDE Program from the Bizkaia County to EB.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Gonzalez-Flores, A., Aguilar-Quesada, R., Siles, E. et al. Interaction between PARP-1 and HIF-2α in the hypoxic response. Oncogene 33, 891–898 (2014). https://doi.org/10.1038/onc.2013.9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.9
Keywords
This article is cited by
-
Crosstalk between hydroxytyrosol, a major olive oil phenol, and HIF-1 in MCF-7 breast cancer cells
Scientific Reports (2020)
-
Targeting Sentinel Proteins and Extrasynaptic Glutamate Receptors: a Therapeutic Strategy for Preventing the Effects Elicited by Perinatal Asphyxia?
Neurotoxicity Research (2018)
-
Reduction of hepatic fibrosis by overexpression of von Hippel–Lindau protein in experimental models of chronic liver disease
Scientific Reports (2017)
-
Vulnerability to a Metabolic Challenge Following Perinatal Asphyxia Evaluated by Organotypic Cultures: Neonatal Nicotinamide Treatment
Neurotoxicity Research (2017)