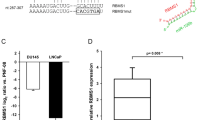
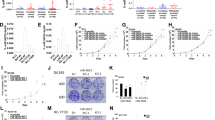
Abstract
The androgen receptor (AR) stimulates and represses gene expression to promote the initiation and progression of prostate cancer. Here, we report that androgen represses the miR-99a/let7c/125b-2 cluster through AR and anti-androgen drugs block the androgen-repression of the miRNA cluster. AR directly binds to the host gene of the miR-99a/let7c/125b-2 cluster, LINC00478. Expression of the cluster is repressed or activated by chromatin remodelers EZH2 or JMJD3 in the presence or absence of androgen, respectively. Bioinformatics analysis reveals a significant enrichment of targets of miR-99a, let-7c and miR-125b in androgen-induced gene sets, suggesting that downregulation of the miR-99a/let7c/125b-2 cluster by androgen protects many of their target mRNAs from degradation and indirectly assists in the gene induction. We validated the hypothesis with 12 potential targets of the miR-99a/let7c/125b-2 cluster induced by androgen: 9 out of the 12 mRNAs are downregulated by the microRNA cluster. To ascertain the biological significance of this hypothesis, we focused on IGF1R, a known prostate cancer growth factor that is induced by androgen and directly targeted by the miR-99a/let7c/125b-2 cluster. The androgen-induced cell proliferation is ameliorated to a similar extent as anti-androgen drugs by preventing the repression of the microRNAs or induction of IGF1R in androgen-dependent prostate cancer cells. Expression of a microRNA-resistant form of IGF1R protects these cells from inhibition by the miR-99a/let7c/125b-2 cluster. These results indicate that a thorough understanding of how androgen stimulates prostate cancer growth requires not only an understanding of genes directly induced/repressed by AR, but also of genes indirectly induced by AR through the repression of key microRNAs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Richter E, Srivastava S, Dobi A . Androgen receptor and prostate cancer. Prostate Cancer Prostatic Dis 2007; 10: 114–118.
Denayer S, Helsen C, Thorrez L, Haelens A, Claessens F . The rules of DNA recognition by the androgen receptor. Mol Endocrinol 2010; 24: 898–913.
Nelson PS, Clegg N, Arnold H, Ferguson C, Bonham M, White J et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci USA 2002; 99: 11890–11895.
Wang Q, Li W, Liu XS, Carroll JS, Janne OA, Keeton EK et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell 2007; 27: 380–392.
Takayama K, Kaneshiro K, Tsutsumi S, Horie-Inoue K, Ikeda K, Urano T et al. Identification of novel androgen response genes in prostate cancer cells by coupling chromatin immunoprecipitation and genomic microarray analysis. Oncogene 2007; 26: 4453–4463.
Massie CE, Adryan B, Barbosa-Morais NL, Lynch AG, Tran MG, Neal DE et al. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep 2007; 8: 871–878.
Gonit M, Zhang J, Salazar M, Cui H, Shatnawi A, Trumbly R et al. Hormone depletion-insensitivity of prostate cancer cells is supported by the AR without binding to classical response elements. Mol Endocrinol 2011; 25: 621–634.
Ngan S, Stronach EA, Photiou A, Waxman J, Ali S, Buluwela L . Microarray coupled to quantitative RT-PCR analysis of androgen-regulated genes in human LNCaP prostate cancer cells. Oncogene 2009; 28: 2051–2063.
Zhao JC, Yu J, Runkle C, Wu L, Hu M, Wu D et al. Cooperation between Polycomb and androgen receptor during oncogenic transformation. Genome Res 2012; 22: 322–331.
Cai C, He HH, Chen S, Coleman I, Wang H, Fang Z et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell 2011; 20: 457–471.
Liu YN, Liu Y, Lee HJ, Hsu YH, Chen JH . Activated androgen receptor downregulates E-cadherin gene expression and promotes tumor metastasis. Mol Cell Biol 2008; 28: 7096–7108.
Jiang J, Pan Y, Regan KM, Wu C, Zhang X, Tindall DJ et al. Androgens repress expression of the F-box protein Skp2 via p107 dependent and independent mechanisms in LNCaP prostate cancer cells. Prostate 2012; 72: 225–232.
Lanzino M, Sisci D, Morelli C, Garofalo C, Catalano S, Casaburi I et al. Inhibition of cyclin D1 expression by androgen receptor in breast cancer cells—identification of a novel androgen response element. Nucleic Acids Res 2010; 38: 5351–5365.
Baniwal SK, Khalid O, Sir D, Buchanan G, Coetzee GA, Frenkel B . Repression of Runx2 by androgen receptor (AR) in osteoblasts and prostate cancer cells: AR binds Runx2 and abrogates its recruitment to DNA. Mol Endocrinol 2009; 23: 1203–1214.
Curtin D, Jenkins S, Farmer N, Anderson AC, Haisenleder DJ, Rissman E et al. Androgen suppression of GnRH-stimulated rat LHbeta gene transcription occurs through Sp1 sites in the distal GnRH-responsive promoter region. Mol Endocrinol 2001; 15: 1906–1917.
Nelius T, Filleur S, Yemelyanov A, Budunova I, Shroff E, Mirochnik Y et al. Androgen receptor targets NFkappaB and TSP1 to suppress prostate tumor growth in vivo. Int J Cancer 2007; 121: 999–1008.
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D et al. MicroRNA expression profiles classify human cancers. Nature 2005; 435: 834–838.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008; 105: 10513–10518.
Sun D, Lee YS, Malhotra A, Kim HK, Matecic M, Evans C et al. miR-99 family of MicroRNAs suppresses the expression of prostate-specific antigen and prostate cancer cell proliferation. Cancer Res 2011; 71: 1313–1324.
Tong AW, Fulgham P, Jay C, Chen P, Khalil I, Liu S et al. MicroRNA profile analysis of human prostate cancers. Cancer Gene Ther 2009; 16: 206–216.
Jalava SE, Urbanucci A, Latonen L, Waltering KK, Sahu B, Janne OA et al. Androgen-regulated miR-32 targets BTG2 and is overexpressed in castration-resistant prostate cancer. Oncogene 2012; 31: 4460–4471.
Waltering KK, Porkka KP, Jalava SE, Urbanucci A, Kohonen PJ, Latonen LM et al. Androgen regulation of micro-RNAs in prostate cancer. Prostate 2011; 71: 604–614.
Wang WL, Chatterjee N, Chittur SV, Welsh J, Tenniswood MP . Effects of 1alpha,25 dihydroxyvitamin D3 and testosterone on miRNA and mRNA expression in LNCaP cells. Mol Cancer 2011; 10: 58.
Gommans WM, Berezikov E . Controlling miRNA regulation in disease. Methods Mol Biol 2012; 822: 1–18.
Ribas J, Ni X, Haffner M, Wentzel EA, Salmasi AH, Chowdhury WH et al. miR-21: an androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res 2009; 69: 7165–7169.
Fletcher CE, Dart DA, Sita-Lumsden A, Cheng H, Rennie PS, Bevan CL . Androgen-regulated processing of the oncomir MiR-27a, which targets Prohibitin in prostate cancer. Hum Mol Genet 2012; 21: 3112–3127.
Chlenski A, Nakashiro K, Ketels KV, Korovaitseva GI, Oyasu R . Androgen receptor expression in androgen-independent prostate cancer cell lines. Prostate 2001; 47: 66–75.
Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 2010; 17: 443–454.
Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 2008; 132: 958–970.
Dahle O, Kumar A, Kuehn MR . Nodal signaling recruits the histone demethylase Jmjd3 to counteract polycomb-mediated repression at target genes. Sci Signal 2010; 3: ra48.
Jung JW, Lee S, Seo MS, Park SB, Kurtz A, Kang SK et al. Histone deacetylase controls adult stem cell aging by balancing the expression of polycomb genes and jumonji domain containing 3. Cell Mol Life Sci 2010; 67: 1165–1176.
Xiang Y, Zhu Z, Han G, Lin H, Xu L, Chen CD . JMJD3 is a histone H3K27 demethylase. Cell Res 2007; 17: 850–857.
Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 2007; 449: 731–734.
Kojima S, Inahara M, Suzuki H, Ichikawa T, Furuya Y . Implications of insulin-like growth factor-I for prostate cancer therapies. Int J Urol 2009; 16: 161–167.
Papatsoris AG, Karamouzis MV, Papavassiliou AG . Novel insights into the implication of the IGF-1 network in prostate cancer. Trends Mol Med 2005; 11: 52–55.
Ozkan EE . Plasma and tissue insulin-like growth factor-I receptor (IGF-IR) as a prognostic marker for prostate cancer and anti-IGF-IR agents as novel therapeutic strategy for refractory cases: a review. Mol Cell Endocrinol 2011; 344: 1–24.
Cardillo MR, Monti S, Di Silverio F, Gentile V, Sciarra F, Toscano V . Insulin-like growth factor (IGF)-I, IGF-II and IGF type I receptor (IGFR-I) expression in prostatic cancer. Anticancer Res 2003; 23: 3825–3835.
Liao Y, Abel U, Grobholz R, Hermani A, Trojan L, Angel P et al. Up-regulation of insulin-like growth factor axis components in human primary prostate cancer correlates with tumor grade. Hum Pathol 2005; 36: 1186–1196.
Gennigens C, Menetrier-Caux C, Droz JP . Insulin-Like Growth Factor (IGF) family and prostate cancer. Crit Rev Oncol Hematol 2006; 58: 124–145.
Antonarakis ES, Carducci MA, Eisenberger MA . Novel targeted therapeutics for metastatic castration-resistant prostate cancer. Cancer Lett 2010; 291: 1–13.
Zhu H, Shyh-Chang N, Segre AV, Shinoda G, Shah SP, Einhorn WS et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 2011; 147: 81–94.
Guan Y, Yao H, Zheng Z, Qiu G, Sun K . MiR-125b targets BCL3 and suppresses ovarian cancer proliferation. Int J Cancer 2011; 128: 2274–2283.
Nadiminty N, Tummala R, Lou W, Zhu Y, Shi XB, Zou JX et al. MicroRNA let-7c is downregulated in prostate cancer and suppresses prostate cancer growth. PLoS One 2012; 7: e32832.
Huang L, Luo J, Cai Q, Pan Q, Zeng H, Guo Z et al. MicroRNA-125b suppresses the development of bladder cancer by targeting E2F3. Int J Cancer 2011; 128: 1758–1769.
Mueller AC, Sun D, Dutta A . The miR-99 family regulates the DNA damage response through its target SNF2H. Oncogene 2013; 32: 1164–1172.
He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y et al. A microRNA component of the p53 tumour suppressor network. Nature 2007; 447: 1130–1134.
Piovan C, Palmieri D, Di Leva G, Braccioli L, Casalini P, Nuovo G et al. Oncosuppressive role of p53-induced miR-205 in triple negative breast cancer. Mol Oncol 2012; 6: 458–472.
O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT . c-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005; 435: 839–843.
Zheng C, Yinghao S, Li J . MiR-221 expression affects invasion potential of human prostate carcinoma cell lines by targeting DVL2. Med Oncol 2012; 29: 815–822.
Grad JM, Dai JL, Wu S, Burnstein KL . Multiple androgen response elements and a Myc consensus site in the androgen receptor (AR) coding region are involved in androgen-mediated up-regulation of AR messenger RNA. Mol Endocrinol 1999; 13: 1896–1911.
Lee JG, Zheng R, McCafferty-Cepero JM, Burnstein KL, Nanus DM, Shen R . Endothelin-1 enhances the expression of the androgen receptor via activation of the c-myc pathway in prostate cancer cells. Mol Carcinog 2009; 48: 141–149.
Nadiminty N, Tummala R, Lou W, Zhu Y, Zhang J, Chen X et al. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J Biol Chem 2012; 287: 1527–1537.
Takayama K, Tsutsumi S, Katayama S, Okayama T, Horie-Inoue K, Ikeda K et al. Integration of cap analysis of gene expression and chromatin immunoprecipitation analysis on array reveals genome-wide androgen receptor signaling in prostate cancer cells. Oncogene 2011; 30: 619–630.
Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR . Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev 2007; 21: 2005–2017.
van Royen ME, van Cappellen WA, de Vos C, Houtsmuller AB, Trapman J . Stepwise androgen receptor dimerization. J Cell Sci 2012; 125 (Pt 8): 1970–1979.
Yu J, Rhodes DR, Tomlins SA, Cao X, Chen G, Mehra R et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Res 2007; 67: 10657–10663.
Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA 2003; 100: 11606–11611.
Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002; 419: 624–629.
Sen GL, Webster DE, Barragan DI, Chang HY, Khavari PA . Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev 2008; 22: 1865–1870.
Ramadoss S, Chen X, Wang CY . Histone demethylase KDM6B promotes epithelial-mesenchymal transition. J Biol Chem 2012; 287: 44508–44517.
Ene CI, Edwards L, Riddick G, Baysan M, Woolard K, Kotliarova S et al. Histone demethylase jumonji D3 (JMJD3) as a tumor suppressor by regulating p53 protein nuclear stabilization. PLoS One 2012; 7: e51407.
Pandini G, Mineo R, Frasca F, Roberts CT, Marcelli M, Vigneri R et al. Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells. Cancer Res 2005; 65: 1849–1857.
Pandini G, Genua M, Frasca F, Vigneri R, Belfiore A . Sex steroids upregulate the IGF-1R in prostate cancer cells through a nongenotropic pathway. Ann NY Acad Sci 2009; 1155: 263–267.
Rubinstein M, Idelman G, Plymate SR, Narla G, Friedman SL, Werner H . Transcriptional activation of the insulin-like growth factor I receptor gene by the Kruppel-like factor 6 (KLF6) tumor suppressor protein: potential interactions between KLF6 and p53. Endocrinology 2004; 145: 3769–3777.
Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR . Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem 2006; 99: 392–401.
Chen Z, Jin Y, Yu D, Wang A, Mahjabeen I, Wang C et al. Down-regulation of the microRNA-99 family members in head and neck squamous cell carcinoma. Oral Oncol 2012; 48: 686–691.
Sun J, Gong X, Purow B, Zhao Z . Uncovering MicroRNA and transcription factor mediated regulatory networks in glioblastoma. PLoS Comput Biol 2012; 8: e1002488.
Magee JA, Chang LW, Stormo GD, Milbrandt J . Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology 2006; 147: 590–598.
Rae JM, Johnson MD, Cordero KE, Scheys JO, Larios JM, Gottardis MM et al. GREB1 is a novel androgen-regulated gene required for prostate cancer growth. Prostate 2006; 66: 886–894.
Wang Y, Li X, Hu H . Transcriptional regulation of co-expressed microRNA target genes. Genomics 2011; 98: 445–452.
Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A . Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol 2006; 174: 677–687.
Qin ZS, Yu J, Shen J, Maher CA, Hu M, Kalyana-Sundaram S et al. HPeak: a Hidden Markov Model-based algorithm for defining read-enriched regions in ChIP-Seq data. BMC Bioinformatics 2010; 11: 369.
Acknowledgements
This work was supported by P01CA104106 from NIH to AD and BMP and DOD PC094499 from the US Army DOD to DS. We thank members of the Prostate Cancer Research Working group at UVA for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Sun, D., Layer, R., Mueller, A. et al. Regulation of several androgen-induced genes through the repression of the miR-99a/let-7c/miR-125b-2 miRNA cluster in prostate cancer cells. Oncogene 33, 1448–1457 (2014). https://doi.org/10.1038/onc.2013.77
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.77
Keywords
This article is cited by
-
Epstein–Barr virus-encoded microRNA BART22 serves as novel biomarkers and drives malignant transformation of nasopharyngeal carcinoma
Cell Death & Disease (2022)
-
Alterations of microRNAs throughout the malignant evolution of cutaneous squamous cell carcinoma: the role of miR-497 in epithelial to mesenchymal transition of keratinocytes
Oncogene (2018)
-
Let-7c inhibits cholangiocarcinoma growth but promotes tumor cell invasion and growth at extrahepatic sites
Cell Death & Disease (2018)
-
Circulating microRNA-99 family as liquid biopsy marker in pancreatic adenocarcinoma
Journal of Cancer Research and Clinical Oncology (2018)
-
The roles of microRNAs in the progression of castration-resistant prostate cancer
Journal of Human Genetics (2017)