Abstract
Reduced expression of the p53 family member p63 has been suggested to play a causative role in cancer metastasis. Here, we show that ΔNp63α, the predominant p63 isoform, plays a major role in regulation of cell migration, invasion and cancer metastasis. We identified mitogen-activated protein (MAP) kinase phosphatase 3 (MKP3) as a downstream target of ΔNp63α that is required for mediating these effects. We show that ΔNp63α regulates extracellular signal-regulated protein kinases 1 and 2 (Erk1/2) activity via MKP3 in both cancer and non-transformed cells. We further show that exogenous ΔNp63α inhibits cell invasion and is dependent on MKP3 upregulation for repression. Conversely, endogenous pan-p63 ablation results in increased cell migration and invasion, which can be reverted by reintroducing the ΔNp63α isoform alone, but not by other isoforms. Interestingly, these effects require Erk2, but not Erk1 expression, and can be rescued by enforced MKP3 expression. Moreover, MKP3 expression is reduced in invasive cancers, and reduced p63 expression increases metastatic frequency in vivo. Taken together, these results suggest an important role for ΔNp63α in preventing cancer metastasis by inhibition of Erk2 signaling via MKP3.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Serber Z, Lai HC, Yang A, Ou HD, Sigal MS, Kelly AE et al. A C-terminal inhibitory domain controls the activity of p63 by an intramolecular mechanism. Mol Cell Biol 2002; 22: 8601–8611.
Thanos CD, Bowie JU . p53 Family members p63 and p73 are SAM domain-containing proteins. Protein Sci 1999; 8: 1708–1710.
Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dötsch V et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 1998; 2: 305–316.
Candi E, Cipollone R, Rivetti di Val Cervo P, Gonfloni S, Melino G, Knight R . p63 in epithelial development. Cell Mol Life Sci 2008; 65: 3126–3133.
Melino G . p63 is a suppressor of tumorigenesis and metastasis interacting with mutant p53. Cell Death Differ 2011; 18: 1487–1499.
Celli J, Duijf P, Hamel BC, Bamshad M, Kramer B, Smits AP et al. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell 1999; 99: 143–153.
McGrath JA, Duijf PH, Doetsch V, Irvine AD, de Waal R, Vanmolkot KR et al. Hay-Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63. Hum Mol Genet 2001; 10: 221–229.
Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999; 398: 714–718.
Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A . p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999; 398: 708–713.
Senoo M, Pinto F, Crum CP, McKeon F . p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell 2007; 129: 523–536.
Shalom-Feuerstein R, Lena AM, Zhou H, De La Forest Divonne S, Van Bokhoven H, Candi E et al. ΔNp63 is an ectodermal gatekeeper of epidermal morphogenesis. Cell Death Differ 2011; 18: 887–896.
Keyes WM, Wu Y, Vogel H, Guo X, Lowe SW, Mills AA . p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes Dev 2005; 19: 1986–1999.
Flores ER, Sengupta S, Miller JB, Newman JJ, Bronson R, Crowley D et al. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell 2005; 7: 363–373.
Su X, Chakravarti D, Cho MS, Liu L, Gi YJ, Lin Y-L et al. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature 2010; 467: 986–990.
Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med 2009; 15: 1179–1185.
Suh E-K, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z et al. p63 protects the female germ line during meiotic arrest. Nature 2006; 444: 624–628.
Candi E, Rufini A, Terrinoni A, Dinsdale D, Ranalli M, Paradisi A et al. Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell Death Differ 2006; 13: 1037–1047.
Carroll DK, Carroll JS, Leong C-O, Cheng F, Brown M, Mills AA et al. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol 2006; 8: 551–561.
Ihrie RA, Marques MR, Nguyen BT, Horner JS, Papazoglu C, Bronson RT et al. Perp is a p63-regulated gene essential for epithelial integrity. Cell 2005; 120: 843–856.
Rocco JW, Leong C-O, Kuperwasser N, DeYoung MP, Ellisen LW . p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 2006; 9: 45–56.
DeYoung MP, Johannessen CM, Leong C-O, Faquin W, Rocco JW, Ellisen LW . Tumor-specific p73 up-regulation mediates p63 dependence in squamous cell carcinoma. Cancer Res 2006; 66: 9362–9368.
Keyes WM, Pecoraro M, Aranda V, Vernersson-Lindahl E, Li W, Vogel H et al. ΔNp63α is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis. Cell Stem Cell 2011; 8: 164–176.
Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE et al. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA 2000; 97: 5462–5467.
Massion PP, Taflan PM, Jamshedur Rahman SM, Yildiz P, Shyr Y, Edgerton ME et al. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res 2003; 63: 7113–7121.
Weber A, Bellmann U, Bootz F, Wittekind C, Tannapfel A . Expression of p53 and its homologues in primary and recurrent squamous cell carcinomas of the head and neck. Int J Cancer 2002; 99: 22–28.
Haqq C, Nosrati M, Sudilovsky D, Crothers J, Khodabakhsh D, Pulliam BL et al. The gene expression signatures of melanoma progression. Proc Natl Acad Sci USA 2005; 102: 6092–6097.
Su H, Hu N, Shih J, Hu Y, Wang Q-H, Chuang EY et al. Gene expression analysis of esophageal squamous cell carcinoma reveals consistent molecular profiles related to a family history of upper gastrointestinal cancer. Cancer Res 2003; 63: 3872–3876.
Vanaja DK, Cheville JC, Iturria SJ, Young CYF . Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res 2003; 63: 3877–3882.
Dumesic PA, Scholl FA, Barragan DI, Khavari PA . Erk1/2 MAP kinases are required for epidermal G2/M progression. J Cell Biol 2009; 185: 409–422.
Yamamoto T, Ebisuya M, Ashida F, Okamoto K, Yonehara S, Nishida E . Continuous ERK activation downregulates antiproliferative genes throughout G1 phase to allow cell-cycle progression. Curr Biol 2006; 16: 1171–1182.
Vicent S, López-Picazo JM, Toledo G, Lozano MD, Torre W, Garcia-Corchón C et al. ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br J Cancer 2004; 90: 1047–1052.
Adeyinka A, Nui Y, Cherlet T, Snell L, Watson PH, Murphy LC . Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin Cancer Res 2002; 8: 1747–1753.
Krueger JS, Keshamouni VG, Atanaskova N, Reddy KB . Temporal and quantitative regulation of mitogen-activated protein kinase (MAPK) modulates cell motility and invasion. Oncogene 2001; 20: 4209–4218.
McCawley LJ, Li S, Wattenberg EV, Hudson LG . Sustained activation of the mitogen-activated protein kinase pathway. A mechanism underlying receptor tyrosine kinase specificity for matrix metalloproteinase-9 induction and cell migration. J Biol Chem 1999; 274: 4347–4353.
Webb CP, Van Aelst L, Wigler MH, Woude GF . Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc Natl Acad Sci USA 1998; 95: 8773–8778.
Ekerot M, Stavridis M, Delavaine L, Mitchell M, Staples C, Owens D et al. Negative feedback regulation of FGF signalling by DUSP6/MKP-3 is driven by ERK1/2 and mediated by Ets factor binding to a conserved site within the DUSP6/MKP-3 gene promoter. Biochem J 2008; 412: 287–298.
Li C, Scott DA, Hatch E, Tian X, Mansour SL . Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development 2007; 134: 167–176.
Fu SW, Schwartz A, Stevenson H, Pinzone JJ, Davenport GJ, Orenstein JM et al. Correlation of expression of BP1, a homeobox gene, with estrogen receptor status in breast cancer. Breast Cancer Res 2003; 5: R82–R87.
Zhao H, Langerød A, Ji Y, Nowels KW, Nesland JM, Tibshirani R et al. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell 2004; 15: 2523–2536.
Hollestelle A, Elstrodt F, Nagel JHA, Kallemeijn WW, Schutte M . Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol Cancer Res 2007; 5: 195–201.
Karlsson M, Mathers J, Dickinson RJ, Mandl M, Keyse SM . Both nuclear-cytoplasmic shuttling of the dual specificity phosphatase MKP-3 and its ability to anchor MAP kinase in the cytoplasm are mediated by a conserved nuclear export signal. J Biol Chem 2004; 279: 41882–41891.
Jinnin M, Ihn H, Mimura Y, Asano Y, Yamane K, Tamaki K . Matrix metalloproteinase-1 up-regulation by hepatocyte growth factor in human dermal fibroblasts via ERK signaling pathway involves Ets1 and Fli1. Nucleic Acids Res 2005; 33: 3540–3549.
Liu S, Liang Y, Huang H, Wang L, Li Y, Li J et al. ERK-dependent signaling pathway and transcriptional factor Ets-1 regulate matrix metalloproteinase-9 production in transforming growth factor-beta1 stimulated glomerular podocytes. Cell Physiol Biochem 2005; 16: 207–216.
Yang S, Zhang JJ, Huang X-Y . Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 2009; 15: 124–134.
Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 2004; 6: 154–161.
Meng L, Gabai VL, Sherman MY . Heat-shock transcription factor HSF1 has a critical role in human epidermal growth factor receptor-2-induced cellular transformation and tumorigenesis. Oncogene 2010; 29: 5204–5213.
Huang C, Jacobson K, Schaller MD . MAP kinases and cell migration. J Cell Sci 2004; 117 (Part 20): 4619–4628.
Shin S, Dimitri CA, Yoon S-O, Dowdle W, Blenis J . ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol Cell 2010; 38: 114–127.
Kuo L, Chang H-C, Leu T-H, Maa M-C, Hung W-C . Src oncogene activates MMP-2 expression via the ERK/Sp1 pathway. J Cell Physiol 2006; 207: 729–734.
Higashikawa K, Yoneda S, Tobiume K, Saitoh M, Taki M, Mitani Y et al. DeltaNp63alpha-dependent expression of Id-3 distinctively suppresses the invasiveness of human squamous cell carcinoma. Int J Cancer 2009; 124: 2837–2844.
Liu J, Zhan M, Hannay JA, Das P, Bolshakov SV, Kotilingam D et al. Wild-type p53 inhibits nuclear factor-kappaB-induced matrix metalloproteinase-9 promoter activation: implications for soft tissue sarcoma growth and metastasis. Mol Cancer Res 2006; 4: 803–810.
Sun Y, Wenger L, Rutter JL, Brinckerhoff CE, Cheung HS . p53 down-regulates human matrix metalloproteinase-1 (Collagenase-1) gene expression. J Biol Chem 1999; 274: 11535–11540.
Fukushima H, Koga F, Kawakami S, Fujii Y, Yoshida S, Ratovitski E et al. Loss of DeltaNp63alpha promotes invasion of urothelial carcinomas via N-cadherin/Src homology and collagen/extracellular signal-regulated kinase pathway. Cancer Res 2009; 69: 9263–9270.
Downward J . Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 2003; 3: 11–22.
Zhang Z, Kobayashi S, Borczuk AC, Leidner RS, Laframboise T, Levine AD et al. Dual specificity phosphatase 6 (DUSP6) is an ETS-regulated negative feedback mediator of oncogenic ERK-signaling in lung cancer cells. Carcinogenesis 2010; 31: 577–586.
Furukawa T, Sunamura M, Motoi F, Matsuno S, Horii A . Potential tumor suppressive pathway involving DUSP6/MKP-3 in pancreatic cancer. Am J Pathol 2003; 162: 1807–1815.
Okudela K, Yazawa T, Woo T, Sakaeda M, Ishii J, Mitsui H et al. Down-regulation of DUSP6 expression in lung cancer: its mechanism and potential role in carcinogenesis. Am J Pathol 2009; 175: 867–881.
Maillet M, Purcell N, Sargent M, York A, Bueno O, Molkentin J . Dusp6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem 2008; 283: 31246–31255.
Barbieri CE, Tang LJ, Brown KA, Pietenpol JA . Loss of p63 leads to increased cell migration and up-regulation of genes involved in invasion and metastasis. Cancer Res 2006; 66: 7589–7597.
Higashikawa K, Yoneda S, Tobiume K, Taki M, Shigeishi H, Kamata N . Snail-induced down-regulation of DeltaNp63alpha acquires invasive phenotype of human squamous cell carcinoma. Cancer Res 2007; 67: 9207–9213.
Kommagani R, Leonard M, Lewis S, Romano R, Sinha S, Kadakia M . Regulation of VDR by {Delta}Np63{alpha} is associated with inhibition of cell invasion. J Cell Sci 2009; 122 (Part 16): 2828–2835.
Lindsay J, McDade SS, Pickard A, McCloskey KD, McCance DJ . The role of {delta}Np63{gamma} in epithelial to mesenchymal transition. J Biol Chem 2011; 286: 3915–3924.
Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell 2009; 137: 87–98.
Muller PAJ, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 2009; 139: 1327–1341.
Eicheler W, Zips D, Dörfler A, Grénman R, Baumann M . Splicing mutations in TP53 in human squamous cell carcinoma lines influence immunohistochemical detection. J Histochem Cytochem 2002; 50: 197–204.
Xu S, Furukawa T, Kanai N, Sunamura M, Horii A . Abrogation of DUSP6 by hypermethylation in human pancreatic cancer. J Hum Genet 2005; 50: 159–167.
Ginos MA, Page GP, Michalowicz BS, Patel KJ, Volker SE, Pambuccian SE et al. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res 2004; 64: 55–63.
Talbot SG, Estilo C, Maghami E, Sarkaria IS, Pham DK, O-charoenrat P et al. Gene expression profiling allows distinction between primary and metastatic squamous cell carcinomas in the lung. Cancer Res 2005; 65: 3063–3071.
Ying H, Chang DLF, Zheng H, McKeon F, Xiao Z-XJ . DNA-binding and transactivation activities are essential for TAp63 protein degradation. Mol Cell Biol 2005; 25: 6154–6164.
Acknowledgements
We thank Dr Frank McKeon (Harvard Medical School, Boston, MA, USA) for providing the myc-tagged murine pcDNA3-ΔNp63α, pcDNA3-ΔNp63γ, pcDNA3-TAp63α and pcDNA3-TAp63γ expression plasmids, and Dr Tyler Jacks (Massachusetts Institute of Technology, Cambridge, MA, USA) for providing a plasmid encoding shRNA against human MKP3. We also thank Dr Xixi Cao (Baylor College of Medicine) for help with Oncomine bioinformatics analysis. This work was supported by NIH grants (CA79804 and GM70017) and by the National Key Basic Research Program (973 Program) of China (2012CB910700) to Z-XJX, and United States Department of Defense Congressionally Directed Medical Research Programs grant (W81XWH-10-1-0161) to JB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Bergholz, J., Zhang, Y., Wu, J. et al. ΔNp63α regulates Erk signaling via MKP3 to inhibit cancer metastasis. Oncogene 33, 212–224 (2014). https://doi.org/10.1038/onc.2012.564
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.564
Keywords
This article is cited by
-
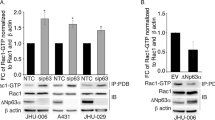
∆Np63α inhibits Rac1 activation and cancer cell invasion through suppression of PREX1
Cell Death Discovery (2024)
-
A network map of cytoskeleton-associated protein 4 (CKAP4) mediated signaling pathway in cancer
Journal of Cell Communication and Signaling (2023)
-
PIK3CA hotspot mutations p. H1047R and p. H1047L sensitize breast cancer cells to thymoquinone treatment by regulating the PI3K/Akt1 pathway
Molecular Biology Reports (2022)
-
ERK3 is transcriptionally upregulated by ∆Np63α and mediates the role of ∆Np63α in suppressing cell migration in non-melanoma skin cancers
BMC Cancer (2021)
-
ΔNp63α down-regulates c-Myc modulator MM1 via E3 ligase HERC3 in the regulation of cell senescence
Cell Death & Differentiation (2018)