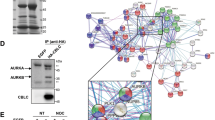
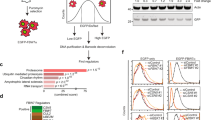
Abstract
Activated Cdc42-associated kinase 1 (ACK1) is a nonreceptor tyrosine kinase linked to cellular transformation. The aberrant regulation of ACK1 promotes tumor progression and metastasis. Therefore, ACK1 is regarded as a valid target in cancer therapy. Seven in absentia homolog (SIAH) ubiquitin ligases facilitate substrate ubiquitinylation that targets proteins to the proteasomal degradation pathway. Here we report that ACK1 and SIAH1 from Homo sapiens interact in a yeast two-hybrid screen. Protein–protein interaction studies and protein degradation analyses using deletion and point mutants of ACK1 verify that SIAH1 and the related SIAH2 interact with ACK1. The association between SIAHs and ACK1 depends on the integrity of a highly conserved SIAH-binding motif located in the far C-terminus of ACK1. Furthermore, we demonstrate that the interaction of ACK1 with SIAH1 and the induction of proteasomal degradation of ACK1 by SIAH1 are independent of ACK1’s kinase activity. Chemical inhibitors blocking proteasomal activity corroborate that SIAH1 and SIAH2 destabilize the ACK1 protein by inducing its proteasomal turnover. This mechanism apparently differs from the lysosomal pathway targeting ACK1 after stimulation with the epidermal growth factor. Our data also show that ACK1, but not ACK1 mutants lacking the SIAH binding motif, has a discernable negative effect on SIAH levels. Additionally, knockdown approaches targeting the SIAH2 mRNA uncover specifically that the induction of SIAH2 expression, by hormonally-induced estrogen receptor (ER) activation, decreases the levels of ACK1 in luminal human breast cancer cells. Collectively, our data provide novel insights into the molecular mechanisms modulating ACK1 and they position SIAH ubiquitin ligases as negative regulators of ACK1 in transformed cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
House CM, Möller A, Bowtell DD . Siah proteins: novel drug targets in the Ras and hypoxia pathways. Cancer Res 2009; 69: 8835–8838.
Krämer OH, Stauber RH, Bug G, Hartkamp J, Knauer SK . SIAH proteins: Critical roles in leukemogenesis. Leukemia 2012, (in press).
Liu M, Hsu J, Chan C, Li Z, Zhou Q . The ubiquitin ligase SIAH1 controls Ell2 stability and formation of super elongation complexes to modulate gene transcription. Mol Cell 2012; 46: 325–334.
Metzger MB, Hristova VA, Weissman AM . HECT and RING finger families of E3 ubiquitin ligases at a glance. J Cell Sci 2012; 125: 531–537.
Mogk A, Schmidt R, Bukau B . The N-end rule pathway for regulated proteolysis: prokaryotic and eukaryotic strategies. Trends Cell Biol 2007; 17: 165–172.
Zhao HL, Ueki N, Hayman MJ . The Ski protein negatively regulates Siah2-mediated HDAC3 degradation. Biochem Biophys Res Commun 2010; 399: 623–628.
Perissi V, Scafoglio C, Zhang J, Ohgi KA, Rose DW, Glass CK et al. TBL1 and TBLR1 phosphorylation on regulated gene promoters overcomes dual CtBP and NCoR/SMRT transcriptional repression checkpoints. Mol Cell 2008; 29: 755–766.
Wu H, Lin Y, Shi Y, Qian W, Tian Z, Yu Y et al. SIAH-1 interacts with mammalian polyhomeotic homologues HPH2 and affects its stability via the ubiquitin-proteasome pathway. Biochem Biophys Res Commun 2010; 397: 391–396.
Frasor J, Danes JM, Funk CC, Katzenellenbogen BS . Estrogen down-regulation of the corepressor N-CoR: mechanism and implications for estrogen derepression of N-CoR-regulated genes. Proc Natl Acad Sci USA 2005; 102: 13153–13157.
Venables JP, Dalgliesh C, Paronetto MP, Skitt L, Thornton JK, Saunders PT et al. SIAH1 targets the alternative splicing factor T-STAR for degradation by the proteasome. Hum Mol Genet 2004; 13: 1525–1534.
Nagano Y, Fukushima T, Okemoto K, Tanaka K, Bowtell DD, Ronai Z et al. Siah1/SIP regulates p27(kip1) stability and cell migration under metabolic stress. Cell Cycle 2011; 10: 2592–2602.
Fukushima T, Zapata JM, Singha NC, Thomas M, Kress CL, Krajewska M et al. Critical function for SIP, a ubiquitin E3 ligase component of the beta-catenin degradation pathway, for thymocyte development and G1 checkpoint. Immunity 2006; 24: 29–39.
Bursen A, Moritz S, Gaussmann A, Dingermann T, Marschalek R . Interaction of AF4 wild-type and AF4.MLL fusion protein with SIAH proteins: indication for t(4;11) pathobiology? Oncogene 2004; 23: 6237–6249.
Habelhah H, Frew IJ, Laine A, Janes PW, Relaix F, Sassoon D et al. Stress-induced decrease in TRAF2 stability is mediated by Siah2. EMBO J 2002; 21: 5756–5765.
Buchwald M, Pietschmann K, Müller JP, Böhmer FD, Heinzel T, Krämer OH . Ubiquitin conjugase UBCH8 targets active FMS-like tyrosine kinase 3 for proteasomal degradation. Leukemia 2010; 24: 1412–1421.
Krämer OH, Müller S, Buchwald M, Reichardt S, Heinzel T . Mechanism for ubiquitylation of the leukemia fusion proteins AML1-ETO and PML-RARalpha. Faseb J 2008; 22: 1369–1379.
Pietschmann K, Buchwald M, Müller S, Knauer SK, Kögl M, Heinzel T et al. Differential regulation of PML-RARalpha stability by the ubiquitin ligases SIAH1/SIAH2 and TRIAD1. Int J Biochem Cell Biol 2012; 44: 132–138.
Pietschmann K, Bolck HA, Buchwald M, Spielberg S, Polzer H, Spiekermann K et al. Breakdown of the FLT3-ITD/STAT5 axis and synergistic apoptosis induction by the histone deacetylase inhibitor Panobinostat and FLT3-specific inhibitors. Mol Cancer Ther 2012, (in press).
Winter M, Sombroek D, Dauth I, Moehlenbrink J, Scheuermann K, Crone J et al. Control of HIPK2 stability by ubiquitin ligase Siah-1 and checkpoint kinases ATM and ATR. Nat Cell Biol 2008; 10: 812–824.
Calzado MA, de la Vega L, Möller A, Bowtell DD, Schmitz ML . An inducible autoregulatory loop between HIPK2 and Siah2 at the apex of the hypoxic response. Nat Cell Biol 2009; 11: 85–91.
Yun S, Möller A, Chae SK, Hong WP, Bae YJ, Bowtell DD et al. Siah proteins induce the epidermal growth factor-dependent degradation of phospholipase Cepsilon. J Biol Chem 2008; 283: 1034–1042.
Wen YY, Yang ZQ, Song M, Li BL, Yao XH, Chen XL et al. The expression of SIAH1 is downregulated and associated with Bim and apoptosis in human breast cancer tissues and cells. Mol Carcinog 2010; 49: 440–449.
Wen YY, Yang ZQ, Song M, Li BL, Zhu JJ, Wang EH . SIAH1 induced apoptosis by activation of the JNK pathway and inhibited invasion by inactivation of the ERK pathway in breast cancer cells. Cancer Sci 2010; 101: 73–79.
Nadeau RJ, Toher JL, Yang X, Kovalenko D, Friesel R . Regulation of Sprouty2 stability by mammalian Seven-in-Absentia homolog 2. J Cell Biochem 2007; 100: 151–160.
Depaux A, Regnier-Ricard F, Germani A, Varin-Blank N . Dimerization of hSiah proteins regulates their stability. Biochem Biophys Res Commun 2006; 348: 857–863.
Xu Z, Sproul A, Wang W, Kukekov N, Greene LA . Siah1 interacts with the scaffold protein POSH to promote JNK activation and apoptosis. J Biol Chem 2006; 281: 303–312.
Ahmed AU, Schmidt RL, Park CH, Reed NR, Hesse SE, Thomas CF et al. Effect of disrupting seven-in-absentia homolog 2 function on lung cancer cell growth. J Natl Cancer Inst 2008; 100: 1606–1629.
Lin Q, Wang J, Childress C, Sudol M, Carey DJ, Yang W . HECTE3 ubiquitin ligase Nedd4-1 ubiquitinates ACK and regulates epidermal growth factor (EGF)-induced degradation of EGF receptor and ACK. Mol Cell Biol 2010; 30: 1541–1554.
Korzeniewski N, Cuevas R, Duensing A, Duensing S . Daughter centriole elongation is controlled by proteolysis. Mol Biol Cell 2010; 21: 3942–3951.
Khurana A, Nakayama K, Williams S, Davis RJ, Mustelin T, Ronai Z . Regulation of the ring finger E3 ligase Siah2 by p38 MAPK. J Biol Chem 2006; 281: 35316–35326.
Mahajan NP, Whang YE, Mohler JL, Earp HS . Activated tyrosine kinase Ack1 promotes prostate tumorigenesis: role of Ack1 in polyubiquitination of tumor suppressor Wwox. Cancer Res 2005; 65: 10514–10523.
Mahajan NP, Liu Y, Majumder S, Warren MR, Parker CE, Mohler JL et al. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc Natl Acad Sci USA 2007; 104: 8438–8443.
Mahajan K, Coppola D, Challa S, Fang B, Chen YA, Zhu W et al. Ack1 mediated AKT/PKB tyrosine 176 phosphorylation regulates its activation. PLoS One 2010; 5: e9646.
Mahajan K, Mahajan NP . Shepherding AKT and androgen receptor by Ack1 tyrosine kinase. J Cell Physiol 2010; 224: 327–333.
House CM, Hancock NC, Möller A, Cromer BA, Fedorov V, Bowtell DD et al. Elucidation of the substrate binding site of Siah ubiquitin ligase. Structure 2006; 14: 695–701.
Twomey E, Li Y, Lei J, Sodja C, Ribecco-Lutkiewicz M, Smith B et al. Regulation of MYPT1 stability by the E3 ubiquitin ligase SIAH2. Exp Cell Res 2010; 316: 68–77.
Yokoyama N, Miller WT . Biochemical properties of the Cdc42-associated tyrosine kinase ACK1. Substrate specificity, authphosphorylation, and interaction with Hck. J Biol Chem 2003; 278: 47713–47723.
Jansen MP, Ruigrok-Ritstier K, Dorssers LC, van Staveren IL, Look MP, Meijer-van Gelder ME et al. Downregulation of SIAH2, an ubiquitin E3 ligase, is associated with resistance to endocrine therapy in breast cancer. Breast Cancer Res Treat 2009; 116: 263–271.
Stebbing J, Filipovic A, Lit LC, Blighe K, Grothey A, Xu Y et al. LMTK3 is implicated in endocrine resistance via multiple signaling pathways. Oncogene 2012, (in press).
Krämer OH, Zhu P, Ostendorff HP, Golebiewski M, Tiefenbach J, Peters MA et al. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. Embo J 2003; 22: 3411–3420.
Lu M, Mira-y-Lopez R, Nakajo S, Nakaya K, Jing Y . Expression of estrogen receptor alpha, retinoic acid receptor alpha and cellular retinoic acid binding protein II genes is coordinately regulated in human breast cancer cells. Oncogene 2005; 24: 4362–4369.
Li C, Lin M, Liu J . Identification of PRC1 as the p53 target gene uncovers a novel function of p53 in the regulation of cytokinesis. Oncogene 2004; 23: 9336–9347.
Holliday DL, Speirs V . Choosing the right cell line for breast cancer research. Breast Cancer Res 2011; 13: 215.
Prieto-Echague V, Miller WT . Regulation of ack-family nonreceptor tyrosine kinases. J Signal Transduct 2011; 2011: 742372.
Pao-Chun L, Chan PM, Chan W, Manser E . Cytoplasmic ACK1 interaction with multiple receptor tyrosine kinases is mediated by Grb2: an analysis of ACK1 effects on Axl signaling. J Biol Chem 2009; 284: 34954–34963.
van der Horst EH, Degenhardt YY, Strelow A, Slavin A, Chinn L, Orf J et al. Metastatic properties and genomic amplification of the tyrosine kinase gene ACK1. Proc Natl Acad Sci USA 2005; 102: 15901–15906.
Mahajan K, Challa S, Coppola D, Lawrence H, Luo Y, Gevariya H et al. Effect of Ack1 tyrosine kinase inhibitor on ligand-independent androgen receptor activity. Prostate 2010; 70: 1274–1285.
Chua BT, Lim SJ, Tham SC, Poh WJ, Ullrich A . Somatic mutation in the ACK1 ubiquitin association domain enhances oncogenic signaling through EGFR regulation in renal cancer derived cells. Mol Oncol 2010; 4: 323–334.
Howlin J, Rosenkvist J, Andersson T . TNK2 preserves epidermal growth factor receptor expression on the cell surface and enhances migration and invasion of human breast cancer cells. Breast Cancer Res 2008; 10: R36.
Mahajan K, Coppola D, Chen YA, Zhu W, Lawrence HR, Lawrence NJ et al. Ack1 tyrosine kinase activation correlates with pancreatic cancer progression. Am J Pathol 2012; 180: 1386–1393.
Chan W, Tian R, Lee YF, Sit ST, Lim L, Manser E . Down-regulation of active ACK1 is mediated by association with the E3 ubiquitin ligase Nedd4-2. J Biol Chem 2009; 284: 8185–8194.
Osborne CK, Neven P, Dirix LY, Mackey JR, Robert J, Underhill C et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin cancer res 2011; 17: 1147–1159.
Sarkar TR, Sharan S, Wang J, Pawar SA, Cantwell CA, Johnson PF et al. Identification of a Src tyrosine kinase/SIAH2 E3 ubiquitin ligase pathway that regulates C/EBPdelta expression and contributes to transformation of breast tumor cells. Mol Cell Biol 2012; 32: 320–332.
Acknowledgements
We thank C. Kosan, M. Korfei and S. Scheiding for their discussions and their excellent help. We are grateful to M. Kögl, N. Varin-Blank, Z. Ronai, R. Marschalek, H. Bursen, A. Baniahmad, O. Werz and O. Huber for providing material. Grant support: German Cancer Aid (FKZ102362); Wilhelm-Sander Foundation (No. 2010.078.1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Buchwald, M., Pietschmann, K., Brand, P. et al. SIAH ubiquitin ligases target the nonreceptor tyrosine kinase ACK1 for ubiquitinylation and proteasomal degradation. Oncogene 32, 4913–4920 (2013). https://doi.org/10.1038/onc.2012.515
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.515
Keywords
This article is cited by
-
Epigenetic reprogramming of cell cycle genes by ACK1 promotes breast cancer resistance to CDK4/6 inhibitor
Oncogene (2023)
-
Inhibiting ACK1-mediated phosphorylation of C-terminal Src kinase counteracts prostate cancer immune checkpoint blockade resistance
Nature Communications (2022)
-
SIAH1 reverses chemoresistance in epithelial ovarian cancer via ubiquitination of YBX-1
Oncogenesis (2022)
-
Inhibitors of class I HDACs and of FLT3 combine synergistically against leukemia cells with mutant FLT3
Archives of Toxicology (2022)
-
Establishment and characterization of HROC69 – a Crohn´s related colonic carcinoma cell line and its matched patient-derived xenograft
Scientific Reports (2016)