Abstract
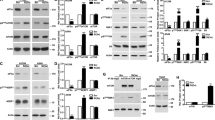
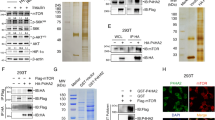
Hypoxia inducible factor-2α (HIF-2α) has a critical role in renal tumorigenesis. HIF-2α is stabilized in von Hippel–Lindau (VHL)-deficient renal cell carcinoma through mechanisms that require ongoing mRNA translation. Mammalian target of rapamycin (mTOR) functions in two distinct complexes: Raptor-associated mTORC1 and Rictor-associated mTORC2. Rictor-associated mTORC2 complex has been linked to maintaining HIF-2α protein in the absence of VHL; however, the mechanisms remain to be elucidated. Although Raptor-associated mTORC1 is a known key upstream regulator of mRNA translation, initiation and elongation, the role of mTORC2 in regulating mRNA translation is not clear. Complex assembly of the mRNA cap protein, eukaryotic translation initiation factor 4 (eIF4)E, with activators (eIF4 gamma (eIF4G)) and inhibitors (eIF4E-binding protein 1 (4E-BP1)) are rate-limiting determinants of mRNA translation. Our laboratory has previously demonstrated that reactive oxygen species, mediated by p22phox-based Nox oxidases, are enhanced in VHL-deficient cells and have a role in the activation of Akt on S473, a site phosphorylated by the mTORC2 complex. In this study, we examined the role of Rictor-dependent regulation of HIF-2α through eIF4E-dependent mRNA translation and examined the effects of p22phox-based Nox oxidases on TORC2 regulation. We demonstrate for the first time that mTORC2 complex stability and activation is redox sensitive, and further defined a novel role for p22phox-based Nox oxidases in eIF4E-dependent mRNA translation through mTORC2. Furthermore, we provide the first evidence that silencing of p22phox reduces HIF-2α-dependent gene targeting in vitro and tumor formation in vivo. The clinical relevance of these studies is demonstrated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kondo K, Kim WY, Lechpammer M, Kaelin WG . Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol 2003; 1: E83.
Kaelin WG . The von Hippel-Lindau tumor suppressor protein and clear cell renal carcinoma. Clin Cancer Res 2007; 13: 680s–684s.
Baldewijns MM, van Vlodrop IJ, Vermeulen PB, Soetekouw PM, van Engeland M, AP de Bruïne . VHL and HIF signalling in renal cell carcinogenesis. J Pathol 2010; 221: 125–138.
Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, Clark RA et al. NAD(P)H oxidases regulate HIF-2alpha protein expression. J Biol Chem 2007; 282: 8019–8026.
Lazaris-Karatzas A, Montine KS, Sonenberg N . Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature 1990; 345: 544–547.
Pandolfi PP . Aberrant mRNA translation in cancer pathogenesis: an old concept revisited comes finally of age. Oncogene 2004; 23: 3134–3137.
Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C et al. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med 2004; 10: 484–486.
Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 2004; 428: 332–327.
Avdulov S, Li S, Michalek V, Burrichter D, Peterson M, Perlman DM et al. Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell 2004; 5: 553–563.
De Benedetti A, Graff JR . eIF-4E expression and its role in malignancies and metastases. Oncogene 2004; 23: 3189–3199.
Topisirovic I, Ruiz-Gutierrez M, Borden KL . Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res 2004; 64: 8639–8642.
Furic L, Rong L, Larsson O, Koumakpayi IH, Yoshida K, Brueschke A et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci USA 2010; 107: 14134–14139.
Hay N, Sonenberg N . Upstream and downstream of mTOR. Genes Dev 2004; 18: 1926–1945.
Guertin DA, Sabatini DM . Defining the role of mTOR in cancer. Cancer Cell 2007; 12: 9–22.
Laplante M, Sabatini DM . mTOR signaling at a glance. J Cell Sci 2009; 122: 3589–3594.
Block K, Gorin Y, New DD, Eid A, Chelmicki T, Reed A et al. The NADPH oxidase subunit p22phox inhibits the function of the tumor suppressor protein tuberin. Am J Pathol 2010; 176: 2447–2455.
Toschi A, Lee E, Gadir N, Ohh M, Foster DA . Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mTORC1 and mTORC2. J Biol Chem 2008; 283: 34495–34499.
Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 2009; 7: e38.
Schalm SS, Fingar DC, Sabatini DM, Blenis J . TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr Biol 2003; 13: 797–806.
Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL . Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J 2008; 27: 1919–1931.
Guertin DA, Stevens DM, Saitoh M, Kinkel S, Crosby K, Sheen JH et al. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell 2009; 15: 148–159.
She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell 2010; 18: 39–51.
Lee HJ, Kim DI, Kang GH, Kwak C, Ku JH, Moon KC . Phosphorylation of ERK1/2 and prognosis of clear cell renal cell carcinoma. Urology 2009; 73: 394–399.
Foster H, Coley HM, Goumenou A, Pados G, Harvey A, Karteris E . Differential expression of mTOR signalling components in drug resistance in ovarian cancer. Anticancer Res 2010; 30: 3529–3534.
Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology 2008; 135: 1972–1983 1983.e1-11.
Guertin DA, Sabatini DM . The pharmacology of mTOR inhibition. Sci Signal 2009; 2: pe24.
Huang J, Dibble CC, Matsuzaki M, Manning BD . The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol 2008; 28: 4104–4115.
Feliers D, Lee MJ, Ghosh-Choudhury G, Bomsztyk K, Kasinath BS . Heterogeneous nuclear ribonucleoprotein K contributes to angiotensin II stimulation of vascular endothelial growth factor mRNA translation. Am J Physiol Renal Physiol 2007; 293: F607–F615.
Acknowledgements
We acknowledge Dr Goutam Gosh-Choudhury for the HRE responsive element, Cynthia Galindo for technical contributions and Dr Hanna E. Abboud for helpful discussions and critical reading of the manuscript. This study was supported by the Veterans Administration Career Development Award and NIH R01 NCI CA131272 (KB), and NIH K08 CA138774 and Voelcker Fund Young Investigator Award (SS). This study was also supported in part by the Cancer Center Support Grant, National Cancer Institute, 5 P30 CA054174-18 (DJP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Nayak, B., Feliers, D., Sudarshan, S. et al. Stabilization of HIF-2α through redox regulation of mTORC2 activation and initiation of mRNA translation. Oncogene 32, 3147–3155 (2013). https://doi.org/10.1038/onc.2012.333
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.333
Keywords
This article is cited by
-
The role of hypoxic signalling in metastasis: towards translating knowledge of basic biology into novel anti-tumour strategies
Clinical & Experimental Metastasis (2018)
-
NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance
Nature Communications (2017)
-
Understanding complexity in the HIF signaling pathway using systems biology and mathematical modeling
Journal of Molecular Medicine (2016)
-
Differential effect of DJ-1/PARK7 on development of natural and induced regulatory T cells
Scientific Reports (2015)
-
Chemotherapy-mediated p53-dependent DNA damage response in clear cell renal cell carcinoma: role of the mTORC1/2 and hypoxia-inducible factor pathways
Cell Death & Disease (2013)