Abstract
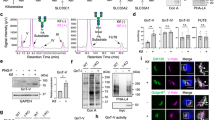
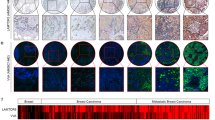
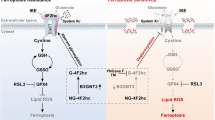
O-linked glycans of secreted and membrane-bound proteins have an important role in the pathogenesis of pancreatic cancer by modulating immune responses, inflammation and tumorigenesis. A critical aspect of O-glycosylation, the position at which proteins are glycosylated with N-acetyl-galactosamine on serine and threonine residues, is regulated by the substrate specificity of UDP-GalNAc:polypeptide N-acetylgalactosaminyl-transferases (GalNAc-Ts). Thus, GalNAc-Ts regulate the first committed step in O-glycosylated protein biosynthesis, determine sites of O-glycosylation on proteins and are important for understanding normal and carcinoma-associated O-glycosylation. We have found that one of these enzymes, GalNAc-T3, is overexpressed in human pancreatic cancer tissues and suppression of GalNAc-T3 significantly attenuates the growth of pancreatic cancer cells in vitro and in vivo. In addition, suppression of GalNAc-T3 induces apoptosis of pancreatic cancer cells. Our results indicate that GalNAc-T3 is likely involved in pancreatic carcinogenesis. Modification of cellular glycosylation occurs in nearly all types of cancer as a result of alterations in the expression levels of glycosyltransferases. We report guanine the nucleotide-binding protein, α-transducing activity polypeptide-1 (GNAT1) as a possible substrate protein of GalNAc-T3. GalNAc-T3 is associated with O-glycosylation of GNAT1 and affects the subcellular distribution of GNAT1. Knocking down endogenous GNAT1 significantly suppresses the growth/survival of PDAC cells. Our results imply that GalNAc-T3 contributes to the function of O-glycosylated proteins and thereby affects the growth and survival of pancreatic cancer cells. Thus, substrate proteins of GalNAc-T3 should serve as important therapeutic targets for pancreatic cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Accession codes
References
Alenzi FQ . (2004). Links between apoptosis, proliferation and the cell cycle. Br J Biomed Sci 61: 1–4.
Andersen MH, Becker JC, Straten P . (2005). Regulators of apoptosis: suitable targets for immune therapy of cancer. Nat Rev Drug Discov 4: 399–409.
Bennett EP, Hassan H, Clausen H . (1996). cDNA cloning and expression of a novel human UDP-N-acetyl-alpha-D-galactosamine. Polypeptide N-acetylgalactosaminyltransferase, GalNAc-T3. J Biol Chem 271: 17006–17012.
Braga VM, Pemberton LF, Duhig T, Gendler SJ . (1992). Spatial and temporal expression of an epithelial mucin, Muc-1, during mouse development. Development 115: 427–437.
Brockhausen I . (1999). Pathways of O-glycan biosynthesis in cancer cells. Biochim Biophys Acta 1473: 67–95.
Cattoretti G, Becker M H, Key G, Duchrow M, Schluter C, Galle J et al. (1992). Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB1 and MIB3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 168: 357–363.
Fuster MM, Esko JD . (2005). The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer 5: 526–542.
Gray JW, Dean PN . (1980). Display and analysis of flow cytometric data. Annu Rev Biophys Bioeng 9: 509–539.
Hakomori S . (1989). Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res 52: 257–331.
Hollingsworth MA, Swanson BJ . (2004). Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4: 45–60.
Iwamura T, Katsuki T, Ide K . (1987). Establishment and characterization of a human pancreatic cancer cell line (SUIT-2) producing carcinoembryonic antigen and carbohydrate antigen 19-9. Jpn J Cancer Res 78: 54–62.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ . (2009). Cancer statistics, 2009. CA Cancer J Clin 59: 225–249.
Jensen ON . (2006). Interpreting the protein language using proteomics. Nat Rev Mol Cell Biol 7: 391–403.
Joshi SS, Kuszynski CA, Bagchi D . (2001). The cellular and molecular basis of health benefits of grape seed proanthocyanidin extract. Curr Pharm Biotechnol 2: 187–200.
Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N . (2004). Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci 95: 377–384.
Kondo A, Li W, Nakagawa T, Nakano M, Koyama N, Wang X et al. (2006). From glycomics to functional glycomics of sugar chains: identification of target proteins with functional changes using gene targeting mice and knock down cells of FUT8 as examples. Biochim Biophys Acta 1764: 1881–1889.
Lee KM, Yasuda H, Hollingsworth MA, Ouellette MM . (2005). Notch2-positive progenitors with the intrinsic ability to give rise to pancreatic ductal cells. Lab Invest 85: 1003–1012.
Li M, Song L, Qin X . (2010). Glycan changes: cancer metastasis and anticancer vaccines. J Biosci 35: 665–673.
Mayoral MA, Mayoral C, Meneses A, Villalvazo L, Guzman A, Espinosa B et al. (2008). Identification of galectin-3 and mucin-type O-glycans in breast cancer and its metastasis to brain. Cancer Invest 26: 615–623.
Mellors A, Lo RY . (1995). O-sialoglycoprotease from Pasteurella haemolytica. Methods Enzymol 248: 728–740.
Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E et al. (2006). Modulation of the Akt/Ras/Raf/MEK/ERK pathway by A(3) adenosine receptor. Purinergic Signal 2: 627–632.
Moniaux N, Andrianifahanana M, Brand RE, Batra SK . (2004). Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer 91: 1633–1638.
Nomoto M, Izumi H, Ise T, Kato K, Takano H, Nagatani G et al. (1999). Structural basis for the regulation of UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyl transferase-3 gene expression in adenocarcinoma cells. Cancer Res 59: 6214–6222.
Reis CA, David L, Correa P, Carneiro F, de Bolos C, Garcia E et al. (1999). Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res 59: 1003–1007.
Rosenzweig DH, Nair KS, Wei J, Wang Q, Garwin G, Saari JC et al. (2007). Subunit dissociation and diffusion determine the subcellular localization of rod and cone transducins. J Neurosci 27: 5484–5494.
Ruiz-Avila L, McLaughlin SK, Wildman D, McKinnon PJ, Robichon A, Spickofsky N et al. (1995). Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. Nature 376: 80–85.
Spiro RG . (2002). Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptides bonds. Glycobiology 12: 43R–56R.
Sutherlin ME, Nishimori I, Caffrey T, Bennett EP, Hassan H, Mandel U et al. (1997). Expression of three UDP-N-acetyl-alpha-D-galactosamine:polypeptide GalNAc N-acetylgalactosaminyltransferases in adenocarcinoma cell lines. Cancer Res 57: 4744–4748.
Takenaka Y, Fukumori T, Raz A . (2004). Galectin-3 and metastasis. Glycoconj J 19: 543–549.
Telford WG, King LE, Fraker PJ . (1991). Evaluation of glucocorticoid-induced DNA fragmentation in mouse thymocytes by flow cytometry. Cell Prolif 24: 447–459.
White T, Bennett EP, Takio K, Sorensen T, Bonding N, Clausen H . (1995). Purification and cDNA cloning of a human UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase. J Biol Chem 270: 24156–24165.
Wray CJ, Ahmad SA, Matthews JB, Lowy AM . (2005). Surgery for pancreatic cancer: recent controversies and current practice. Gastroenterology 128: 1626–1641.
Acknowledgements
We thank Michel Ouellette for providing the HPNE cells and Tamotsu Takeuchi for supporting pathological experiments and helpful discussion. We also thank Janice Taylor and James Talaska for excellent technical assistance. This work was funded by the NIH with grants to MAH (U01CA111294; R01CA057362).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Taniuchi, K., Cerny, R., Tanouchi, A. et al. Overexpression of GalNAc-transferase GalNAc-T3 promotes pancreatic cancer cell growth. Oncogene 30, 4843–4854 (2011). https://doi.org/10.1038/onc.2011.194
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.194
Keywords
This article is cited by
-
Inter- and intratumoral proteomics and glycosaminoglycan characterization of ALK rearranged lung adenocarcinoma tissues: a pilot study
Scientific Reports (2023)
-
Emerging glyco-risk prediction model to forecast response to immune checkpoint inhibitors in colorectal cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Depletion of eukaryotic initiation factor 5B (eIF5B) reprograms the cellular transcriptome and leads to activation of endoplasmic reticulum (ER) stress and c-Jun N-terminal kinase (JNK)
Cell Stress and Chaperones (2021)
-
Mucins as anti-cancer targets: perspectives of the glycobiologist
Glycoconjugate Journal (2021)
-
Long non-coding RNA-SNHG7 acts as a target of miR-34a to increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in colorectal cancer progression
Journal of Hematology & Oncology (2018)