Abstract
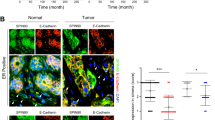
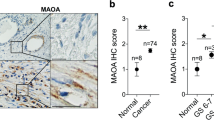
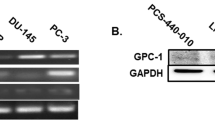
The interaction between cancer cells and microenvironment has a critical role in tumor development and progression. Although microRNAs regulate all the major biological mechanisms, their influence on tumor microenvironment is largely unexplored. Here, we investigate the role of microRNAs in the tumor-supportive capacity of stromal cells. We demonstrated that miR-15 and miR-16 are downregulated in fibroblasts surrounding the prostate tumors of the majority of 23 patients analyzed. Such downregulation of miR-15 and miR-16 in cancer-associated fibroblasts (CAFs) promoted tumor growth and progression through the reduced post-transcriptional repression of Fgf-2 and its receptor Fgfr1, which act on both stromal and tumor cells to enhance cancer cell survival, proliferation and migration. Moreover, reconstitution of miR-15 and miR-16 impaired considerably the tumor-supportive capability of stromal cells in vitro and in vivo. Our data suggest a molecular circuitry in which miR-15 and miR-16 and their correlated targets cooperate to promote tumor expansion and invasiveness through the concurrent activity on stromal and cancer cells, thus providing further support to the development of therapies aimed at reconstituting miR-15 and miR-16 in advanced prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Acevedo VD, Ittmann M, Spencer DM . (2009). Paths of FGFR-driven tumorigenesis. Cell Cycle 8: 580–588.
Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW et al. (2007). Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res 67: 4244–4253.
Aqeilan RI, Calin GA, Croce CM . (2010). miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ 17: 215–220.
Bandi N, Zbinden S, Gugger M, Arnold M, Kocher V, Hasan L et al. (2009). miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res 69: 5553–5559.
Bartel DP . (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297.
Bhattacharya R, Nicoloso M, Arvizo R, Wang E, Cortez A, Rossi S et al. (2009). MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Res 69: 9090–9095.
Bhowmick NA, Neilson EG, Moses HL . (2004). Stromal fibroblasts in cancer initiation and progression. Nature 432: 332–337.
Bonci D, Cittadini A, Latronico MV, Borello U, Aycock JK, Drusco A et al. (2003). Advanced’ generation lentiviruses as efficient vectors for cardiomyocyte gene transduction in vitro and in vivo. Gene Ther 10: 630–636.
Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L et al. (2008). The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med 14: 1271–1277.
Calin GA, Croce CM . (2006). MicroRNA-cancer connection: the beginning of a new tale. Cancer Res 66: 7390–7394.
Chung LW, Chang SM, Bell C, Zhau HE, Ro JY, von Eschenbach AC . (1989). Co-inoculation of tumorigenic rat prostate mesenchymal cells with non-tumorigenic epithelial cells results in the development of carcinosarcoma in syngeneic and athymic animals. Int J Cancer 43: 1179–1187.
Chung LW, Cunha GR . (1983). Stromal-epithelial interactions: II. Regulation of prostatic growth by embryonic urogenital sinus mesenchyme. Prostate 4: 503–511.
Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M et al. (2005). miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA 102: 13944–13949.
Cronauer MV, Hittmair A, Eder IE, Hobisch A, Culig Z, Ramoner R et al. (1997). Basic fibroblast growth factor levels in cancer cells and in sera of patients suffering from proliferative disorders of the prostate. Prostate 31: 223–233.
Cunha GR, Chung LW, Shannon JM, Taguchi O, Fujii H . (1983). Hormone-induced morphogenesis and growth: role of mesenchymal-epithelial interactions. Recent Prog Horm Res 39: 559–598.
De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG et al. (2007). Inflammation in prostate carcinogenesis. Nat Rev Cancer 7: 256–269.
Efstathiou E, Logothetis CJ . (2010). A new therapy paradigm for prostate cancer founded on clinical observations. Clin Cancer Res 16: 1100–1107.
Giri D, Ropiquet F, Ittmann M . (1999). Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res 5: 1063–1071.
Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD et al. (2001). Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res 61: 8135–8142.
He QM, Wei YQ, Tian L, Zhao X, Su JM, Yang L et al. (2003). Inhibition of tumor growth with a vaccine based on xenogeneic homologous fibroblast growth factor receptor-1 in mice. J Biol Chem 278: 21831–21836.
Iliopoulos D, Hirsch HA, Struhl K . (2009). An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 139: 693–706.
Josson S, Matsuoka Y, Chung LW, Zhau HE, Wang R . (2010). Tumor-stroma co-evolution in prostate cancer progression and metastasis. Semin Cell Dev Biol 21: 26–32.
Joyce JA, Pollard JW . (2009). Microenvironmental regulation of metastasis. Nat Rev Cancer 9: 239–252.
Kalluri R, Weinberg RA . (2009). The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428.
Kaminski A, Hahne JC, Haddouti el M, Florin A, Wellmann A, Wernert N . (2006). Tumour-stroma interactions between metastatic prostate cancer cells and fibroblasts. Int J Mol Med 18: 941–950.
Karaa ZS, Iacovoni JS, Bastide A, Lacazette E, Touriol C, Prats H . (2009). The VEGF IRESes are differentially susceptible to translation inhibition by miR-16. RNA 15: 249–254.
Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T et al. (2010). The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell 17: 28–40.
Konig A, Menzel T, Lynen S, Wrazel L, Rosen A, Al-Katib A et al. (1997). Basic fibroblast growth factor (bFGF) upregulates the expression of bcl-2 in B cell chronic lymphocytic leukemia cell lines resulting in delaying apoptosis. Leukemia 11: 258–265.
Kwabi-Addo B, Ozen M, Ittmann M . (2004). The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer 11: 709–724.
Lewis BP, Burge CB, Bartel DP . (2005). Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20.
Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB . (2003). Prediction of mammalian microRNA targets. Cell 115: 787–798.
Menzel T, Rahman Z, Calleja E, White K, Wilson EL, Wieder R et al. (1996). Elevated intracellular level of basic fibroblast growth factor correlates with stage of chronic lymphocytic leukemia and is associated with resistance to fludarabine. Blood 87: 1056–1063.
Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK et al. (1997). Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276: 955–960.
Nakamoto T, Chang CS, Li AK, Chodak GW . (1992). Basic fibroblast growth factor in human prostate cancer cells. Cancer Res 52: 571–577.
Navarro A, Diaz T, Gallardo E, Vinolas N, Marrades RM, Gel B et al. (2011). Prognostic implications of miR-16 expression levels in resected non-small-cell lung cancer. J Surg Oncol 103: 411–415.
Polnaszek N, Kwabi-Addo B, Peterson LE, Ozen M, Greenberg NM, Ortega S et al. (2003). Fibroblast growth factor 2 promotes tumor progression in an autochthonous mouse model of prostate cancer. Cancer Res 63: 5754–5760.
Polyak K, Weinberg RA . (2009). Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9: 265–273.
Risbridger GP, Taylor RA . (2008). Minireview: regulation of prostatic stem cells by stromal niche in health and disease. Endocrinology 149: 4303–4306.
Roccaro AM, Sacco A, Thompson B, Leleu X, Azab AK, Azab F et al. (2009). MicroRNAs 15a and 16 regulate tumor proliferation in multiple myeloma. Blood 113: 6669–6680.
Sahni A, Simpson-Haidaris PJ, Sahni SK, Vaday GG, Francis CW . (2008). Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2). J Thromb Haemost 6: 176–183.
Sezer O, Jakob C, Eucker J, Niemoller K, Gatz F, Wernecke K et al. (2001). Serum levels of the angiogenic cytokines basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in multiple myeloma. Eur J Haematol 66: 83–88.
Shin VY, Jin H, Ng EK, Cheng AS, Chong WW, Wong CY et al. (2011). NF-κB targets miR-16 and miR-21 in gastric cancer: involvement of prostaglandin E receptors. Carcinogenesis 32: 240–245.
Smith JA, Madden T, Vijjeswarapu M, Newman RA . (2001). Inhibition of export of fibroblast growth factor-2 (FGF-2) from the prostate cancer cell lines PC3 and DU145 by Anvirzel and its cardiac glycoside component, oleandrin. Biochem Pharmacol 62: 469–472.
Song S, Wientjes MG, Gan Y, Au JL . (2000). Fibroblast growth factors: an epigenetic mechanism of broad spectrum resistance to anticancer drugs. Proc Natl Acad Sci USA 97: 8658–8663.
Thiery JP . (2002). Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2: 442–454.
Thiery JP, Acloque H, Huang RY, Nieto MA . (2009). Epithelial-mesenchymal transitions in development and disease. Cell 139: 871–890.
Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA et al. (2010). FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res 70: 2085–2094.
Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR . (2002). Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 8: 2912–2923.
van Moorselaar RJ, Voest EE . (2002). Angiogenesis in prostate cancer: its role in disease progression and possible therapeutic approaches. Mol Cell Endocrinol 197: 239–250.
Wernert N, Kaminski A, Haddouti el M, Hahne JC . (2007). Tumor-stroma interactions of metastatic prostate cancer cell lines: analyses using microarrays. Methods Mol Biol 382: 223–237.
Winter SF, Acevedo VD, Gangula RD, Freeman KW, Spencer DM, Greenberg NM . (2007). Conditional activation of FGFR1 in the prostate epithelium induces angiogenesis with concomitant differential regulation of Ang-1 and Ang-2. Oncogene 26: 4897–4907.
Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW . (1994). Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer 57: 406–412.
Yang F, Strand DW, Rowley DR . (2008). Fibroblast growth factor-2 mediates transforming growth factor-beta action in prostate cancer reactive stroma. Oncogene 27: 450–459.
Zhang Y, Song S, Yang F, Au JL, Wientjes MG . (2001). Nontoxic doses of suramin enhance activity of doxorubicin in prostate tumors. J Pharmacol Exp Ther 299: 426–433.
Acknowledgements
We thank Giuseppe Loreto and Maria Rita Pulvirenti for technical assistance. This work was supported by the Italian Health Ministry with ‘Under forty researchers 2007’and Italy-USA programs and by the Italian Association for Cancer Research (AIRC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Musumeci, M., Coppola, V., Addario, A. et al. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene 30, 4231–4242 (2011). https://doi.org/10.1038/onc.2011.140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.140