Abstract
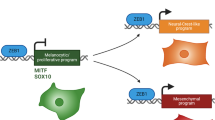
In melanoma, as well as in other solid tumors, the cells within a given tumor exhibit strong morphological, functional and molecular heterogeneity that might reflect the existence of different cancer cell populations, among which are melanoma-initiating cells (MICs) with ‘stemness’ properties and their differentiated, fast-growing progeny. The existence of a slow-growing population might explain the resistance of melanoma to classical chemotherapies that target fast growing cells. Therefore, elucidating the biologic properties of MICs and, more importantly, the molecular mechanisms that drive the transition between MICs and their proliferating progeny needs to be addressed to develop an efficient melanoma therapy. Using B16 mouse melanoma cells and syngeneic mice, we show that the inhibition of microphthalmia-associated transcription factor (Mitf), the master regulator of melanocyte differentiation, increases the tumorigenic potential of melanoma cells and upregulates the stem cell markers Oct4 and Nanog. Notably, p27, the CDK inhibitor, is increased in Mitf-depleted cells and is required for exacerbation of the tumorigenic properties of melanoma cells. Further, a slow-growing population with low-Mitf level and high tumorigenic potential exists spontaneously in melanoma. Ablation of this population dramatically decreases tumor formation. Importantly, these data were confirmed using human melanoma cell lines and freshly isolated human melanoma cell from lymph node and skin melanoma metastasis. Taken together our data, identified Mitf and p27 as the key molecular switches that control the transition between MICs and their differentiated progeny. Eradication of low-Mitf cells might be an appealing strategy to cure melanoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- CFSE:
-
carboxyfluorescein diacetate succinimidyl ester
- MICs:
-
melanoma-initiating cells
- Mitf:
-
microphthalmia-associated transcription factor
- Oct4:
-
octamer-binding protein 4
- TGFβ:
-
transforming growth factor beta
References
Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP et al. (1998). Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol 142: 827–835.
Besson A, Hwang HC, Cicero S, Donovan SL, Gurian-West M, Johnson D et al. (2007). Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev 21: 1731–1746.
Botton T, Puissant A, Bahadoran P, Annicotte JS, Fajas L, Ortonne JP et al. (2009). In vitro and in vivo anti-melanoma effects of ciglitazone. J Invest Dermatol 129: 1208–1218.
Busca R, Ballotti R . (2000). Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res 13: 60–69.
Carreira S, Goodall J, Denat L, Rodriguez M, Nuciforo P, Hoek KS et al. (2006). Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev 20: 3426–3439.
Cheli Y, Ohanna M, Ballotti R, Bertolotto C . (2010). Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res 23: 27–40.
Dalerba P, Clarke MF . (2007). Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell 1: 241–242.
Du J, Widlund HR, Horstmann MA, Ramaswamy S, Ross K, Huber WE et al. (2004). Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by Mitf. Cancer Cell 6: 565–576.
Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S et al. (2005). A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 65: 9328–9337.
Goodall J, Carreira S, Denat L, Kobi D, Davidson I, Nuciforo P et al. (2008). Brn-2 represses microphthalmia-associated transcription factor expression and marks a distinct subpopulation of microphthalmia-associated transcription factor-negative melanoma cells. Cancer Res 68: 7788–7794.
Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA et al. (2009). Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138: 645–659.
Hoek KS, Eichhoff OM, Schlegel NC, Dobbeling U, Kobert N, Schaerer L et al. (2008). In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res 68: 650–656.
Hoek KS, Goding CR . (2010). Cancer stem cells versus phenotype-switching in melanoma. Pigment Cell Melanoma Res 23: 746–759.
Hu Y, Smyth GK . (2009). ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods 347: 70–78.
Larribere L, Hilmi C, Khaled M, Gaggioli C, Bille K, Auberger P et al. (2005). The cleavage of microphthalmia-associated transcription factor, Mitf, by caspases plays an essential role in melanocyte and melanoma cell apoptosis. Genes Dev 19: 1980–1985.
Larribere L, Khaled M, Tartare-Deckert S, Busca R, Luciano F, Bille K et al. (2004). PI3K mediates protection against TRAIL-induced apoptosis in primary human melanocytes. Cell Death Differ 11: 1084–1091.
Larue L, Delmas V . (2006). The WNT/Beta-catenin pathway in melanoma. Front Biosci 11: 733–742.
Lyons AB, Hasbold J, Hodgkin PD . (2001). Flow cytometric analysis of cell division history using dilution of carboxyfluorescein diacetate succinimidyl ester, a stably integrated fluorescent probe. Methods Cell Biol 63: 375–398.
Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y et al. (2010). Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell 6: 130–140.
Nowell PC . (1976). The clonal evolution of tumor cell populations. Science 194: 23–28.
Pinner S, Jordan P, Sharrock K, Bazley L, Collinson L, Marais R et al. (2009). Intravital imaging reveals transient changes in pigment production and Brn2 expression during metastatic melanoma dissemination. Cancer Res 69: 7969–7977.
Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ . (2008). Efficient tumour formation by single human melanoma cells. Nature 456: 593–598.
Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A et al. (2010). A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell 141: 583–594.
Schouwey K, Beermann F . (2008). The Notch pathway: hair graying and pigment cell homeostasis. Histol Histopathol 23: 609–619.
Spira AI, Carducci MA . (2003). Differentiation therapy. Curr Opin Pharmacol 3: 338–343.
Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V et al. (2007). Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA 104: 5895–5900.
Strizzi L, Abbott DE, Salomon DS, Hendrix MJ . (2008). Potential for cripto-1 in defining stem cell-like characteristics in human malignant melanoma. Cell Cycle 7: 1931–1935.
Yang G, Li Y, Nishimura EK, Xin H, Zhou A, Guo Y et al. (2008). Inhibition of PAX3 by TGF-beta modulates melanocyte viability. Mol Cell 32: 554–563.
Acknowledgements
We wish to thank Genevieve Gozzerino, Agnès Loubat and Karine Bille for their help. This work was supported by the ‘Association pour la Recherche contre le Cancer’ (grant ARC 4985) and YC and TB have been supported by ARC's fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Cheli, Y., Guiliano, S., Botton, T. et al. Mitf is the key molecular switch between mouse or human melanoma initiating cells and their differentiated progeny. Oncogene 30, 2307–2318 (2011). https://doi.org/10.1038/onc.2010.598
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.598
Keywords
This article is cited by
-
Novel cellular systems unveil mucosal melanoma initiating cells and a role for PI3K/Akt/mTOR pathway in mucosal melanoma fitness
Journal of Translational Medicine (2024)
-
Delineating the early dissemination mechanisms of acral melanoma by integrating single-cell and spatial transcriptomic analyses
Nature Communications (2023)
-
The microphthalmia-associated transcription factor is involved in gastrointestinal stromal tumor growth
Cancer Gene Therapy (2023)
-
Acetylation reprograms MITF target selectivity and residence time
Nature Communications (2023)
-
A unique hyperdynamic dimer interface permits small molecule perturbation of the melanoma oncoprotein MITF for melanoma therapy
Cell Research (2023)