Abstract
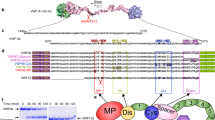
Factor XI (FXI), a coagulation protein essential to normal hemostasis, circulates as a disulfide-linked dimer. Here we report the full-length FXI zymogen crystal structure, revealing that the protease and four apple domains assemble into a unique 'cup and saucer' architecture. The structure shows that the thrombin and platelet glycoprotein Ib binding sites are remote within the monomer but lie in close proximity across the dimer, suggesting a transactivation mechanism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Walsh, P.N. in Hemostasis and Thrombosis: Basic Principles & Clinical Practice 4th edn. (eds. Colman, R.W., Hirsh, J., Marder, V.J., Clowes, A.W. & George, J.N.) 191–202 (Lippincott Williams & Wilkins, Philadelphia, 2001).
Fujikawa, K., Chung, D.W., Hendrickson, L.E. & Davie, E.W. Biochemistry 25, 2417–2424 (1986).
Baglia, F.A., Gailani, D., Lopez, J.A. & Walsh, P.N. J. Biol. Chem. 279, 45470–45476 (2004).
Yun, T.H. et al. J. Biol. Chem. 278, 48112–48119 (2003).
Baglia, F.A., Badellino, K.O., Ho, D.H., Dasari, V.R. & Walsh, P.N. J. Biol. Chem. 275, 31954–31962 (2000).
Baglia, F.A., Jameson, B.A. & Walsh, P.N. J. Biol. Chem. 267, 4247–4252 (1992).
Sinha, D., Seaman, F.S. & Walsh, P.N. Biochemistry 26, 3768–3775 (1987).
Sun, M.F., Zhao, M. & Gailani, D. J. Biol. Chem. 274, 36373–36378 (1999).
Lietha, D., Chirgadze, D.Y., Mulloy, B., Blundell, T.L. & Gherardi, E. EMBO J. 20, 5543–5555 (2001).
Navaneetham, D. et al. J. Biol. Chem. 280, 36165–36175 (2005).
Friedrich, R. et al. Nature 425, 535–539 (2003).
Baglia, F.A. et al. J. Biol. Chem. 279, 49323–49329 (2004).
Acknowledgements
We are grateful to E. Mitchell at European Synchrotron Research Facility beamline ID23 for assistance in data collection. This work was funded by grants from the US National Institutes of Health (RO1 HL46213, PO1 HL74124, Project 3) to P.N.W. and from the British Heart Foundation (PG/04/129/18095) and the Wellcome Trust (072975) to J.E.
Author information
Authors and Affiliations
Contributions
E.P. performed crystallization, structure determination and model building. P.A.M. performed model refinement. P.N.W. contributed FXI protein samples. J.E. contributed to each stage of the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Stereo diagrams of the FXI structure and representative electron density (PDF 664 kb)
Supplementary Fig. 2
FXI interdomain contacts (PDF 764 kb)
Supplementary Fig. 3
Charged surface representation of the FXI dimer (PDF 461 kb)
Supplementary Table 1
Data collection and refinement statistics (PDF 88 kb)
Rights and permissions
About this article
Cite this article
Papagrigoriou, E., McEwan, P., Walsh, P. et al. Crystal structure of the factor XI zymogen reveals a pathway for transactivation. Nat Struct Mol Biol 13, 557–558 (2006). https://doi.org/10.1038/nsmb1095
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1095
This article is cited by
-
Factor XI: structure, function and therapeutic inhibition
Journal of Thrombosis and Thrombolysis (2024)
-
A systematic approach for evaluating the role of surface-exposed loops in trypsin-like serine proteases applied to the 170 loop in coagulation factor VIIa
Scientific Reports (2022)
-
The many faces of the contact pathway and their role in thrombosis
Journal of Thrombosis and Thrombolysis (2011)
-
Antithrombotic Therapy in Acute Coronary Syndrome: How Far Up the Coagulation Cascade Will We Go?
Current Cardiology Reports (2010)