Abstract
ABC-F proteins have evaded functional characterization even though they compose one of the most widely distributed branches of the ATP-binding cassette (ABC) superfamily. Herein, we demonstrate that YjjK, the most prevalent eubacterial ABC-F protein, gates ribosome entry into the translation elongation cycle through a nucleotide-dependent interaction sensitive to ATP/ADP ratio. Accordingly, we rename this protein energy-dependent translational throttle A (EttA). We determined the crystal structure of Escherichia coli EttA and used it to design mutants for biochemical studies including enzymological assays of the initial steps of protein synthesis. These studies suggest that EttA may regulate protein synthesis in energy-depleted cells, which have a low ATP/ADP ratio. Consistently with this inference, EttA-deleted cells exhibit a severe fitness defect in long-term stationary phase. These studies demonstrate that an ABC-F protein regulates protein synthesis via a new mechanism sensitive to cellular energy status.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others

References
Davidson, A.L., Dassa, E., Orelle, C. & Chen, J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 72, 317–364 (2008).
Cavanaugh, L.F., Palmer, A.G. III, Gierasch, L.M. & Hunt, J.F. Disorder breathes life into a DEAD motor. Nat. Struct. Mol. Biol. 13, 566–569 (2006).
Jones, P.M. & George, A.M. Subunit interactions in ABC transporters: towards a functional architecture. FEMS Microbiol. Lett. 179, 187–202 (1999).
Hopfner, K.P. et al. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101, 789–800 (2000).
Smith, P.C. et al. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol. Cell 10, 139–149 (2002).
Holland, I.B. & Blight, M.A. ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J. Mol. Biol. 293, 381–399 (1999).
Jaciuk, M., Nowak, E., Skowronek, K., Tanska, A. & Nowotny, M. Structure of UvrA nucleotide excision repair protein in complex with modified DNA. Nat. Struct. Mol. Biol. 18, 191–197 (2011).
Lammens, K. et al. The Mre11:Rad50 structure shows an ATP-dependent molecular clamp in DNA double-strand break repair. Cell 145, 54–66 (2011).
Skogerson, L. & Wakatama, E. A ribosome-dependent GTPase from yeast distinct from elongation factor 2. Proc. Natl. Acad. Sci. USA 73, 73–76 (1976).
Khoshnevis, S. et al. The iron-sulphur protein RNase L inhibitor functions in translation termination. EMBO Rep. 11, 214–219 (2010).
Pisarev, A.V. et al. The role of ABCE1 in eukaryotic posttermination ribosomal recycling. Mol. Cell 37, 196–210 (2010).
Barthelme, D. et al. Ribosome recycling depends on a mechanistic link between the FeS cluster domain and a conformational switch of the twin-ATPase ABCE1. Proc. Natl. Acad. Sci. USA 108, 3228–3233 (2011).
Becker, T. et al. Structural basis of highly conserved ribosome recycling in eukaryotes and archaea. Nature 482, 501–506 (2012).
Kamath, A. & Chakraburtty, K. Role of yeast elongation factor 3 in the elongation cycle. J. Biol. Chem. 264, 15423–15428 (1989).
Andersen, C.B. et al. Structure of eEF3 and the mechanism of transfer RNA release from the E-site. Nature 443, 663–668 (2006).
Kurata, S. et al. Ribosome recycling step in yeast cytoplasmic protein synthesis is catalyzed by eEF3 and ATP. Proc. Natl. Acad. Sci. USA 107, 10854–10859 (2010).
Tyzack, J.K., Wang, X., Belsham, G.J. & Proud, C.G. ABC50 interacts with eukaryotic initiation factor 2 and associates with the ribosome in an ATP-dependent manner. J. Biol. Chem. 275, 34131–34139 (2000).
Paytubi, S. et al. ABC50 promotes translation initiation in mammalian cells. J. Biol. Chem. 284, 24061–24073 (2009).
Kiel, M.C., Aoki, H. & Ganoza, M.C. Identification of a ribosomal ATPase in Escherichia coli cells. Biochimie 81, 1097–1108 (1999).
Babu, M. et al. Ribosome-dependent ATPase interacts with conserved membrane protein in Escherichia coli to modulate protein synthesis and oxidative phosphorylation. PLoS ONE 6, e18510 (2011).
Kerr, I.D. Sequence analysis of twin ATP binding cassette proteins involved in translational control, antibiotic resistance, and ribonuclease L inhibition. Biochem. Biophys. Res. Commun. 315, 166–173 (2004).
Punta, M. et al. The Pfam protein families database. Nucleic Acids Res. 40, D290–D301 (2012).
Vazquez de Aldana, C.R., Marton, M.J. & Hinnebusch, A.G. GCN20, a novel ATP binding cassette protein, and GCN1 reside in a complex that mediates activation of the eIF-2 alpha kinase GCN2 in amino acid-starved cells. EMBO J. 14, 3184–3199 (1995).
Sattlegger, E. & Hinnebusch, A.G. Polyribosome binding by GCN1 is required for full activation of eukaryotic translation initiation factor 2α kinase GCN2 during amino acid starvation. J. Biol. Chem. 280, 16514–16521 (2005).
Dong, J., Lai, R., Jennings, J.L., Link, A.J. & Hinnebusch, A.G. The novel ATP-binding cassette protein ARB1 is a shuttling factor that stimulates 40S and 60S ribosome biogenesis. Mol. Cell Biol. 25, 9859–9873 (2005).
Hopkins, J.D., Clements, M. & Syvanen, M. New class of mutations in Escherichia coli (uup) that affect precise excision of insertion elements and bacteriophage Mu growth. J. Bacteriol. 153, 384–389 (1983).
Murat, D., Bance, P., Callebaut, I. & Dassa, E. ATP hydrolysis is essential for the function of the Uup ATP-binding cassette ATPase in precise excision of transposons. J. Biol. Chem. 281, 6850–6859 (2006).
Murat, D., Goncalves, L. & Dassa, E. Deletion of the Escherichia coli uup gene encoding a protein of the ATP binding cassette superfamily affects bacterial competitiveness. Res. Microbiol. 159, 671–677 (2008).
Lu, P., Vogel, C., Wang, R., Yao, X. & Marcotte, E.M. Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nat. Biotechnol. 25, 117–124 (2007).
Chen, B. et al. EttA regulates translation by binding to the ribosomal E site and restricting ribosome-tRNA dynamics. Nat. Struct. Mol. Biol. doi:10.1038/nsmb.2741 (5 January 2014).
Zaitseva, J., Jenewein, S., Jumpertz, T., Holland, I.B. & Schmitt, L. H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J. 24, 1901–1910 (2005).
Karcher, A., Schele, A. & Hopfner, K.P. X-ray structure of the complete ABC enzyme ABCE1 from Pyrococcus abyssi. J. Biol. Chem. 283, 7962–7971 (2008).
Oldham, M.L. & Chen, J. Crystal structure of the maltose transporter in a pretranslocation intermediate state. Science 332, 1202–1205 (2011).
Diederichs, K. et al. Crystal structure of MalK, the ATPase subunit of the trehalose/maltose ABC transporter of the archaeon Thermococcus litoralis. EMBO J. 19, 5951–5961 (2000).
Karpowich, N. et al. Crystal structures of the MJ1267 ATP binding cassette reveal an induced-fit effect at the ATPase active site of an ABC transporter. Structure 9, 571–586 (2001).
Vergani, P., Lockless, S.W., Nairn, A.C. & Gadsby, D.C. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature 433, 876–880 (2005).
Fei, J. et al. A highly purified, fluorescently labeled in vitro translation system for single-molecule studies of protein synthesis. Methods Enzymol. 472, 221–259 (2010).
Yusupova, G.Z., Yusupov, M.M., Cate, J.H. & Noller, H.F. The path of messenger RNA through the ribosome. Cell 106, 233–241 (2001).
Youngman, E.M., Brunelle, J.L., Kochaniak, A.B. & Green, R. The active site of the ribosome is composed of two layers of conserved nucleotides with distinct roles in peptide bond formation and peptide release. Cell 117, 589–599 (2004).
Fei, J., Kosuri, P., MacDougall, D.D. & Gonzalez, R.L. Jr. Coupling of ribosomal L1 stalk and tRNA dynamics during translation elongation. Mol. Cell 30, 348–359 (2008).
Agrawal, R.K., Heagle, A.B., Penczek, P., Grassucci, R.A. & Frank, J. EF-G-dependent GTP hydrolysis induces translocation accompanied by large conformational changes in the 70S ribosome. Nat. Struct. Biol. 6, 643–647 (1999).
Aleksandrov, A.A., Cui, L. & Riordan, J.R. Relationship between nucleotide binding and ion channel gating in cystic fibrosis transmembrane conductance regulator. J. Physiol. (Lond.) 587, 2875–2886 (2009).
Ramakrishnan, V. Ribosome structure and the mechanism of translation. Cell 108, 557–572 (2002).
Buckstein, M.H., He, J. & Rubin, H. Characterization of nucleotide pools as a function of physiological state in Escherichia coli. J. Bacteriol. 190, 718–726 (2008).
Glembotski, C.C., Chapman, A.G. & Atkinson, D.E. Adenylate energy charge in Escherichia coli CR341T28 and properties of heat-sensitive adenylate kinase. J. Bacteriol. 145, 1374–1385 (1981).
Lu, Q. & Inouye, M. Adenylate kinase complements nucleoside diphosphate kinase deficiency in nucleotide metabolism. Proc. Natl. Acad. Sci. USA 93, 5720–5725 (1996).
Bernard, M.A., Ray, N.B., Olcott, M.C., Hendricks, S.P. & Mathews, C.K. Metabolic functions of microbial nucleoside diphosphate kinases. J. Bioenerg. Biomembr. 32, 259–267 (2000).
Walton, G.M. & Gill, G.N. Nucleotide regulation of protein synthesis. Methods Enzymol. 60, 578–590 (1979).
Fei, J., Richard, A.C., Bronson, J.E. & Gonzalez, R.L. Jr. Transfer RNA–mediated regulation of ribosome dynamics during protein synthesis. Nat. Struct. Mol. Biol. 18, 1043–1051 (2011).
Tran, Q.H. & Unden, G. Changes in the proton potential and the cellular energetics of Escherichia coli during growth by aerobic and anaerobic respiration or by fermentation. Eur. J. Biochem. 251, 538–543 (1998).
Swedes, J.S., Sedo, R.J. & Atkinson, D.E. Relation of growth and protein synthesis to the adenylate energy charge in an adenine-requiring mutant of Escherichia coli. J. Biol. Chem. 250, 6930–6938 (1975).
Jewett, M.C., Miller, M.L., Chen, Y. & Swartz, J.R. Continued protein synthesis at low [ATP] and [GTP] enables cell adaptation during energy limitation. J. Bacteriol. 191, 1083–1091 (2009).
Chapman, A.G., Fall, L. & Atkinson, D.E. Adenylate energy charge in Escherichia coli during growth and starvation. J. Bacteriol. 108, 1072–1086 (1971).
Gaal, T., Bartlett, M.S., Ross, W., Turnbough, C.L. & Gourse, R.L. Transcription regulation by initiating NTP Concentration: rRNA synthesis in bacteria. Science 278, 2092–2097 (1997).
Walton, G.M. & Gill, G.N. Regulation of ternary (Met-tRNAf - GTP - eukaryotic initiation factor 2) protein synthesis initiation complex formation by the adenylate energy charge. Biochim. Biophys. Acta 418, 195–203 (1976).
Schifano, J.M. et al. Mycobacterial toxin MazF-mt6 inhibits translation through cleavage of 23S rRNA at the ribosomal A site. Proc. Natl. Acad. Sci. USA 110, 8501–8506 (2013).
Yamaguchi, Y., Park, J.H. & Inouye, M. Toxin-antitoxin systems in bacteria and archaea. Annu. Rev. Genet. 45, 61–79 (2011).
Häuser, R. et al. RsfA (YbeB) proteins are conserved ribosomal silencing factors. PLoS Genet. 8, e1002815 (2012).
Polikanov, Y.S., Blaha, G.M. & Steitz, T.A. How hibernation factors RMF, HPF, and YfiA turn off protein synthesis. Science 336, 915–918 (2012).
Yamagishi, M. et al. Regulation of the Escherichia coli rmf gene encoding the ribosome modulation factor: growth phase- and growth rate-dependent control. EMBO J. 12, 625–630 (1993).
Berlyn, M.B. & Letovsky, S. Genome-related datasets within the E. coli Genetic Stock Center database. Nucleic Acids Res. 20, 6143–6151 (1992).
Miller, J.H. Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria (Cold Spring Harbor Laboratory Press, Plainview, New York, 1992).
Datsenko, K.A. & Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97, 6640–6645 (2000).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006).
Neidhardt, F.C. & Curtiss, R. Escherichia coli and Salmonella: Cellular and Molecular Biology (ASM Press, Washington, DC, 1996).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Terwilliger, T.C. & Berendzen, J. Automated MAD and MIR structure solution. Acta Crystallogr. D Biol. Crystallogr. 55, 849–861 (1999).
Terwilliger, T.C. Maximum-likelihood density modification using pattern recognition of structural motifs. Acta Crystallogr. D Biol. Crystallogr. 57, 1755–1762 (2001).
Jones, T.A., Zou, J.-Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Brünger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Kleywegt, G.J. Quality control and validation. Methods Mol. Biol. 364, 255–272 (2007).
Hirashima, A. & Inouye, M. Specific biosynthesis of an envelope protein of Escherichia coli. Nature 242, 405–407 (1973).
Christensen-Dalsgaard, M. & Gerdes, K. Two higBA loci in the Vibrio cholerae superintegron encode mRNA cleaving enzymes and can stabilize plasmids. Mol. Microbiol. 62, 397–411 (2006).
Shimizu, Y. et al. Cell-free translation reconstituted with purified components. Nat. Biotechnol. 19, 751–755 (2001).
Levin, P.A. in Methods in Microbiology Vol. 31 (ed. Philippe Sansonetti, A.Z.) 115–132 (Academic Press, 2002).
Ron, E.Z., Kohler, R.E. & Davis, B.D. Polysomes extracted from Escherichia coli by freeze-thaw-lysozyme lysis. Science 153, 1119–1120 (1966).
Acknowledgements
This work was supported by US National Science Foundation grants to J.F.H. (0424043) and R.L.G. (MCB CAREER 0644262), a Burroughs Wellcome Fund award to R.L.G. (CABS 1004856), a Canadian Institutes of Health Research grant to H.-J.W. (MOP 114938), a grant from the US National Institutes of Health (NIH) Protein Structure Initiative to the Northeast Structural Genomics Consortium (GM074958) and NIH grants to R.L.G. (GM084288) and to J.F. (GM29169 and GM55440). M.T.E. was supported by the NIH Training Program in Molecular Biophysics at Columbia University (T32 GM008281). J.F. is supported as an Investigator by the Howard Hughes Medical Institute. The authors thank J. Hurley and N. Woychik of the University of Medicine and Dentistry of the State of New Jersey for assistance with in vivo radiolabeling, A. Tzagoloff for sharing equipment and the members of the Hunt and Gonzalez laboratories for advice and technical assistance.
Author information
Authors and Affiliations
Contributions
P.C.S. determined the crystal structure and performed the polysome analysis of WT EttA. J.J.F. performed the ATPase measurements. G.B., with assistance from A.J.T., performed the other biochemical and genetic studies. W.N. performed the smFRET experiments. M.T.E. provided training and reagents for in vitro translation assays and eTLC analysis of in vitro translation products. B.C., Y.H. and J.F. determined in the cryo-EM structure of ribosome-bound EttA-EQ2. G.B., P.C.S., H.-J.W., R.L.G. and J.F.H. designed the experiments. G.B., P.C.S., B.C., J.F., R.L.G. and J.F.H. conceived the research program and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Structure-based ABC-F sequence alignment.
The secondary structural elements observed in the crystal structure of E. coli EttA are shown above the sequence alignment, with α–helices represented by cylinders, β–strands by arrows, and 310 helices by circles. Secondary structure elements are colored as in Fig. 2a in the main text. The disordered protein segments are represented by dotted lines. The alignment shows EttA orthologues from E. coli (ECOLI), A. tumefaciens (AGRT5), M. tuberculosis (MYCTU) and B. subtillis (BACSU), as well as the three additional ABC-F proteins from E. coli (YheS, Uup, and YbiT), the three ABC-F in humans (ABCF1, 2 and 3), the two ABC-F (ARB1 and GCN20) and two other non-ABC-F soluble ABC (EF3A and ABCE1/RNaseLI (RLI1)) from S. cerevisiae (yeast). The alignment was initially generated using CLUSTAL-Ω via the Uniprot website (http://www.uniprot.org/) and then manually edited using Jalview to correctly align the Q loop motifs in all sequences and to accurately represent the structural alignment of EttA to the other ABC domains of known structure. The alignment is colored according to the default CLUSTAL coloring code. Large insertions compared to EttA were truncated, and the number of residues removed at each such site is indicated in red and flanked by “+” signs in the text of the alignment.
Supplementary Figure 2 Topology diagram of EttA.
Secondary structure elements are colored as in Fig. 2a, with β–strands represented by arrows, α–helices represented by cylinders, and turns of 310 helix represented by circles. The locations of the Walker A, Walker B, and ABC Signature (LSGGQ/E) motifs involved in ATP binding are indicated by thick dashed lines. The locations of disordered segments in the crystal structure are also indicated.
Supplementary Figure 3 Variations in ABCα subdomain alignment in the crystal structure of the E. coli EttA dimer.
(a) Stereopair showing the molecular surface of a model for the ATP-bound conformation of ABC1 and ABC2 in EttA, which was produced by applying a rigid-body rotation to one protomer in the crystallographically observed EttA dimer (as described in the legend for Fig. 2b in the main text). The structure is colored according to subdomain in an equivalent manner to Fig. 2 in the main text and Supplemental Fig. 1-2 above. The ATP molecules shown in gray space-filling representation are from the Na-ATP-bound MJ0796 dimer, which was used to model the ATP-bound EttA conformation as described above. This surface representation demonstrates that the ATP-binding site on the right, which is at the interface of the ABCα subdomain in ABC1 and the ABCβ and F1-like core subdomains in ABC2, is more open that the ATP-binding site on the left, which is formed by the ABCα subdomain in ABC2 and the ABCβ and F1-like core subdomains in ABC1. This asymmetry is attributable primarily to the different alignments of the ABCα subdomain relative to the F1-like core subdomain in ABC1 vs. ABC2, as shown in panels b-c below in this figure. (b,c) Stereo ribbon diagrams showing ABC1 (panel b) or ABC2 (panel c) from EttA superimposed on a protomer from the Na-ATP-bound dimer of the E171Q mutant of MJ0796 (PDB id 1L2T), based on least-squares alignment of the ABCβ and F1-like core subdomains. The EttA subdomains are colored like panel a above, while MJ076 is colored pink. These images show that, in the nucleotide-free crystal structure of EttA, the ABCα and F1-like core subdomains within each ABC domain are rotated relative to one another by 18-20° compared to their alignment in the canonical ATP-binding conformation. Similar reorientations of these subdomains have been observed in other ABC domain structures that do not have the γ-phosphate group of ATP bound in the active site.3,4. Formation of the catalytically active complex upon ATP binding to EttA presumably involves relative rotation of the ABCα and F1-like core subdomains within both the ABC1 and ABC2 domains as well as a mutual rotation of these domains (as modeled in Fig. 2c) to bring them into the canonical ATP-sandwich conformation observed in the ATP-bound structures of other ABC proteins 5,6,7. (d) Observed apo structure and modeled ATP-bound conformation of the E. coli EttA dimer. Stereo ribbon diagrams of a model for the ATP-bound (solid colors) conformation superimposed on the crystallographically observed nucleotide-free conformation (translucent colors), based on least-squares alignment of the B protomers. The crystallographically observed apo structure (Table 1) was used to model the ATP-bound conformation by aligning the ABCβ and F1-like core subdomains in ABC1 and ABC2 in different EttA protomers to the protomers in the Na-ATP-bound dimer of the E171Q mutant of MJ0796 (PDB id 1L2T). ATP molecules from the MJ0796 dimer are represented in gray space-filling representation. The structures are colored as in Fig. 2 in the main text and oriented such that the interface of one of the two ABC1-ABC2 interfaces in the EttA dimer is visible in each of the two views, which are related by a 90° rotation around a vertical axis.
Supplementary Figure 4 Conformational change in the PtIM in the EttA monomer versus dimer and the packing interactions of the C terminus of PtIM.
These stereopairs show the molecular surface of ABC2 and a ribbon representation of ABC1 and the PtIM (i.e., the interdomain linker between ABC1 and ABC2), colored like Fig. 2 in the main text. (a) Crystallographically observed apo conformation of one ABC1-ABC2 domain pair in the EttA dimer (Supplementary Fig. 3 and Table 1). This image was generated as described for Fig. 2b in the main text, i.e., by deletion of all the residues prior to the Lys 286 in the protomer A and deletion of all the residues after the Gln 278 in the protomer B. (b) ATP-bound conformation of the EttA monomer modeled from the cryo-EM structure of EttA-EQ2 bound to 70S ribosomes, as presented in the accompanying paper 8. Two α-helices from the C-terminal half of the PtIM that pack onto the surface of ABC2 in the dimer structure (panel a) must undergo a conformational change to enable ABC1 and ABC2 in a single protomer to interact with one another in proper geometry in the EttA monomer (panel b). The C-terminal segment of the PtIM (i.e., the segment after that undergoing the conformational change but before the start of ABC2) packs into a crevice at the interface between the ABCβ subdomain and the F1-like core subdomain in ABC2. The crystallographically observed conformation of these C-terminal residues in the PtIM could modulate the relative rotation of ABCα and F1-like core subdomains required to adopt the catalytically active nucleotide-sandwich conformation (described in Supplemental Fig. S3b-c). The cryo-EM structure of ribosome-bound EttA8 shows that the latter two α-helices in the PtIM refold compared to their crystallographically observed conformation to form a long α–helical hairpin in conjunction with the first α–helix in the PtIM (panel a vs. b), which remains intact. This conformational change in the PtIM is likely to be coupled to dissociation of the EttA dimer that is observed in its crystal structure.
Supplementary Figure 5 Further characterization of EttA in vitro and in vivo.
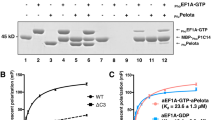
(a) Gel filtration analysis of purified E. coli EttA shows a slow but reversible monomer-dimer equilibrium. Analytical gel-filtration chromatography on a system equipped with in-line UV, refractive index (RI), and static light-scattering detectors (SLS) detectors was used to analyze two samples of WT EttA at different concentrations. A protein fraction from the leading edge of the peak from a preparative gel-filtration column, representing the final step in the purification of EttA, was either injected directly onto an analytical gel-filtration column (red trace) or diluted 1:1 in the elution buffer and heated for 2 hours at 37 °C prior to injection onto the same analytical gel-filtration column (blue trace). The insert shows equivalently color-coded plots of the RI (left axis and dotted lines) and calculated molar mass (right axis and solid lines) vs. volume for these two chromatograms. The same volume of protein solution (100 μl) was injected for both of the analytical gel filtration runs displayed here. Debye analyses of the SLS/RI data indicate that the single peak in the black trace and the earlier peak in the gray trace have a mass-averaged molecular weight of ~126 kDa, consistent with the presence of an EttA dimerA (based on the 62.4 kDa predicted molecular weight of the protomer). Debye analysis of the second peak in the gray trace indicates a mass-averaged molecular weights of ~64 kDa, consistent with the presence of an EttA monomer. Concentration of this monomer peak and re-injection on an analytical gel-filtration column shows a mixture of monomer and dimer. Analytical gel filtration chromatography was conduced on a Shodex 804 column running at 4 °C with a 0.5 ml/min flow-rate in 150 mM NaCl, 5% glycerol, 20mM Tris-HCL, pH 7.2. SLS and RI detectors were from Wyatt Technology. (b) E. coli EttA protein co-fractionates with polysomes. An extract of mid-log phase cultures of E. coli was fractionated using sucrose density gradient sedimentation either without (black trace and upper gel) or with (red trace and lower gel) RNaseA treatment. The optical density of the gradients at 254 nm is shown above immunoblots of the corresponding fractions developed using a polyclonal anti-EttA antiserum. RNaseA treatment cleaves the mRNA holding the polysomes together to yield single fully assembled ribosomes (containing both large and small subunits) that sediment at 70S. (c) Analysis of variations in EttA expression level during the E. coli life cycle. WT or ΔettA MG1655 cells were inoculated into fresh LB medium, and the cultures were monitored during 24 hours of growth at 37 °C. The graph is showing the OD600 of the MG1655 culture in gray (right axis) and the intensity of the signal on the immunoblot in green (left axis). The near-IR fluorescence recorded from the immunoblot was quantified using ImageJ9 software. Insert, Western blot analysis of total cells extracts using a polyclonal anti-EttA antibody, after growth for the number of hours indicated at the top of the blot. (d) Stimulation of EttA ATPase activity by 70S ribosome. Initial velocity of ATP hydrolysis (vertical axis) was measured as a function of ATP concentration (horizontal axis) for WT EttA alone or in the presence of 70S E. coli ribosomes. Assays were performed in polymix buffer (5 mM Tris-acetate (pH 7.5), 95 mM KCl, 5 mM NH4Cl, 5 mM Mg(OAc)2, 0.5 mM CaCl2, 2 mM 2-mercaptoethanol, 5 mM putrescine, and 1 mM spermidine).
Supplementary Figure 6 EttA-EQ2 inhibits protein translation in vitro in complete and minimum translation assays.
(a) Luminescence assays were used to quantify the level of in vitro translation of luciferase from T7-luc mRNA using a complete in vitro translation system, specifically the E. coli S30 Extract System for Linear Templates (Promega). Briefly, reactions containing the indicated EttA variant (or an equivalent volume of buffer) were started by adding T7-luc mRNA and incubated at 37 °C for 2 hours prior to conducting luciferase activity assays using a luminometer. Background from a reaction without T7-luc mRNA was subtracted from the values reported here. See the Methods section for details. (b) Equivalent luminescence assay were used to quantify the level of in vitro translation of luciferase from the same mRNA template using a minimal in vitro translation system, specifically the PURExpress System (New England Biolabs). These reactions were conducted in triplicate in 96-well plates, which were incubated at 37 °C for 2 hours prior to conducting luminescence assays using a microplate reader. (c) Autoradiography was used to assay in vitro translation of the bacteriophage T4 pg32 protein using the PURExpress system (New England Biolabs). EttA variants were added to a final concentration of 5 μM, and reactions programmed with pT7gp32.1-224 mRNA were incubated at 37 °C for 2 hours prior to autoradiography of samples run a 12.5% (w/v) SDS-PAGE gel. Each EttA variant was added either before the mRNA (labeled “1st”), immediately after the mRNA (labeled “2nd”), or 15 min after addition of the mRNA (labeled “at 15'”).
Supplementary Figure 7 Order-of-addition experiments demonstrate that EttA-EQ2 acts after 70S IC formation to trap ribosomes after dipeptide formation and that it can stimulate peptide-bond formation on the ribosome.
Minimum in vitro translation assays were conducted as in Fig. 4 in the main text in the presence of 0.5 mM Mg-ATP but with variations in the timing of addition of some components, as illustrated in the schematic diagrams on the left in panels a-c. Ribosomally synthesized peptides, up to 4 amino acids in length, were separated and quantified by autoradiography of eTLC plates, as shown on the right in panels a-c. (a) Addition of EttA-EQ2 before or after formation of the 70S IC produces an equivalent inhibition of translation following dipeptide formation. The tripeptide synthesis experiment shown here, in which EttA was added before 70S IC formation, gives equivalent results to the experiment shown in Fig. 4a in the main text, in which EttA was added after 70S IC formation. Translation reactions were started by adding the elongation factors and Phe-tRNAPhe and Lys-tRNALys. (b) Addition of EF-G prior to EttA-EQ2 reduces the extent of inhibition of protein translation but does not lead to strong accumulation of tripeptide in a tetrapeptide synthesis assay. Translation reactions containing EF-G were set up to stop after dipeptide synthesis due to lack of a cognate tRNA for the third codon in the model mRNA. Inclusion of EF-G in these reactions should result in translocation of the dipeptide-bearing tRNA to the P site in a substantial fraction of ribosomes. Subsequent addition of EttA-EQ2 inhibits dipeptide elongation in these assays much less effectively than in assays in which translocation of the dipeptide-bearing tRNA has been prevented by omission of EF-G prior to the addition of EttA (Fig. 4b in main), showing that it acts preferentially on the pretranslocation complex with deacylated tRNAfmet in the A site of the ribosome. Moreover, tetrapeptide is formed in higher yield than tripeptide in these assays in which EF-G is added to translation reactions before EttA-EQ2, demonstrating that it inhibits elongation of dipeptides more strongly than that of tripeptides, again consistent with it acting preferentially on ribosomes bearing tRNAfmet in the P site. (c) Addition of EttA-EQ2 after tripeptide synthesis on the ribosome complex modestly stimulates tetrapeptide synthesis rather than inhibiting it. Translation reactions were set up in presence of EF-G with cognate tRNAs for the first three but not the fourth codon in the mRNA, thereby stopping translation after tripeptide synthesis. EttA-EQ2 was subsequently added prior to addition of the cognate tRNA for the fourth codon. The modest stimulation of tetrapeptide synthesis in this experiment provides evidence that interaction of the ATP-bound conformation of EttA with ribosomes promotes peptide-bond formation in the peptidyl-transferase center. The results of this experiment also support the conclusion that EttA-EQ2 does not inhibit post-translocation ribosomal complexes because it specifically blocks the translocation step in the ribosomal elongation cycle. (d) Filter-binding assay showing that the ribosomal E site does not stably retain deacylated tRNAs at the Mg2+ concentration used in standard translation assays. The retention of 0.4 μM deacylated [32P]tRNAPhe by 70S ribosomes was assayed in the presence of different concentrations of Mg(OAc)2 (3.5, 10, and 20 mM). Assays were conducted using a 0.2 μM concentration of 70S ribosomes in 0.1 M NH4Cl, 20 mM Tris-HCl, pH 7.4. At 3.5 mM Mg2+, which is the concentration used in the minimum in vitro translation reactions, the deacylated tRNA is not retained on the E site of the ribosome, even through it is retained at the higher Mg2+ concentrations. This observation indicates that EttA-EQ2 should have access to the E site in the post-translocation complex prepared in the experiment shown in panel c, consistent with the interpretation that binding of EttA-EQ2 at this site stimulates peptide bond formation in the peptidyl-transferase center on the ribosome.
Supplementary Figure 8 Characterization of EttA interactions with the 70S IC with the smFRETL1–L9 signal.
(a) Cartoon diagram of the 70S IC used in these experiments. The 30S and 50S subunits of the ribosome are shown in tan and blue, respectively. The fMet-tRNAfMet is represented by a green ribbon, while the mRNA is represented by a black curve. The Cy3 donor and Cy5 acceptor fluorophores are represented by green and red circles, respectively. (b-d) Data from smFRETL1-L9 experiments recorded in the presence of either 2 mM Mg-ATP (top panels) or 2 mM Mg-ADP (bottom panels) and either in the absence of EttA (panel b), in the presence of 6 μM WT-EttA (panel c), or in the presence of 6 μM EttA-EQ2 (panel d). These surface contour plots show the time evolution of the FRET efficiency (EFRET) distribution in an ensemble of individually observed 70S ICs. The plots were generated by superimposing a large set of individual EFRET versus time trajectories as previously described10; the variable N shown on each plot indicates the number of superimposed trajectories. The contours are color-coded as calibrated by the color bars shown on the right, with white and red representing the lowest and highest populated EFRET levels, respectively. (e) Vertical column scatter plots showing the mean EFRET values measured in a series of independent smFRETL1-L9 experiments conducted in the presence of either Mg-ATP (top panel, three independent experiments) or Mg-ADP (bottom panel, five independent experiments). A plot encompassing the full EFRET range (0.0–1.0) is shown on the left side of each panel, while an expanded view of the relevant EFRET range (0.5–0.6) is shown on the right side of each panel. Independent experiments in the absence or presence of WT-EttA were recorded in a paired fashion (i.e., with data collected from the same flowcell before and after adding WT-EttA). The mean EFRET values from experiments conducted in the same flowcell are shown in the same color. Independent experiments in the absence or presence of EttA-EQ2 were recorded in an unpaired fashion (i.e., with data collected from different flowcells). The back horizontal lines on the graphs represent the mean EFRET value for each experimental condition, while the p-values for the observed differences between the experimental conditions are shown at the top of each scatter plot. The p-values were calculated in version 5 of the program PRISM (Graphpad Inc.) using a paired t-test for WT-EttA and an unpaired t-test for EttA-EQ2.
Supplementary Figure 9 Full view of the cropped eTLC and gels presented in Figures 4 and 6.
(a) Miniature cropped eTLC presented in figures 4a (left) and corresponding uncropped eTLC (left) the red rectangle delimit the area presented on the left. (b) Miniature cropped eTLC presented in figures 4b (left) and corresponding uncropped eTLC (left) the red rectangles delimit the two areas (1 and 2) presented on the left. (c) Miniature cropped eTLC presented in figures 4c (left) and corresponding uncropped eTLC (left) the red rectangles delimit the two areas (1 and 2) presented on the left. (d) Miniature cropped DNA agarose gels presented in figures 6 (top) and corresponding uncropped DNA agarose gels (center and bottom). The red rectangles delimit the 6 areas (1 to 6 in red) presented in the miniature of figure 6 (top).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9, Supplementary Table 1 and Supplementary Note (PDF 5691 kb)
Rights and permissions
About this article
Cite this article
Boël, G., Smith, P., Ning, W. et al. The ABC-F protein EttA gates ribosome entry into the translation elongation cycle. Nat Struct Mol Biol 21, 143–151 (2014). https://doi.org/10.1038/nsmb.2740
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2740
This article is cited by
-
Regulation of the macrolide resistance ABC-F translation factor MsrD
Nature Communications (2023)
-
Interplay between an ATP-binding cassette F protein and the ribosome from Mycobacterium tuberculosis
Nature Communications (2022)
-
The Vibrio vulnificus stressosome is an oxygen-sensor involved in regulating iron metabolism
Communications Biology (2022)
-
CRISPRi chemical genetics and comparative genomics identify genes mediating drug potency in Mycobacterium tuberculosis
Nature Microbiology (2022)
-
Insights into genome recoding from the mechanism of a classic +1-frameshifting tRNA
Nature Communications (2021)