Abstract
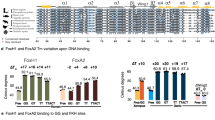
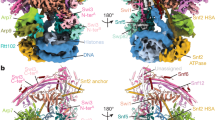
Absent, small or homeotic discs–like 2 (ASH2L) is a trithorax group (TrxG) protein and a regulatory subunit of the SET1 family of lysine methyltransferases. Here we report that ASH2L binds DNA using a forkhead-like helix-wing-helix (HWH) domain. In vivo, the ASH2L HWH domain is required for binding to the β-globin locus control region, histone H3 Lys4 (H3K4) trimethylation and maximal expression of the β-globin gene (Hbb-1), validating the functional importance of the ASH2L DNA binding domain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Dou, Y. et al. Nat. Struct. Mol. Biol. 13, 713–719 (2006).
Steward, M.M. et al. Nat. Struct. Mol. Biol. 13, 852–854 (2006).
Rampalli, S. et al. Nat. Struct. Mol. Biol. 14, 1150–1156 (2007).
Tan, C.C. et al. Proc. Natl. Acad. Sci. USA 105, 7472–7477 (2008).
Demers, C. et al. Mol. Cell 27, 573–584 (2007).
Cao, F. et al. PLoS ONE 5, e14102 (2010).
Avdic, V. et al. Structure 19, 101–108 (2011).
Holm, L., Kaariainen, S., Rosenstrom, P. & Schenkel, A. Bioinformatics 24, 2780–2781 (2008).
Obsil, T. & Obsilova, V. Biochim. Biophys. Acta published online, doi:10.1016/j.bbamcr.2010.11.025 (10 December 2010).
Obsil, T. & Obsilova, V. Oncogene 27, 2263–2275 (2008).
Brent, M.M., Anand, R. & Marmorstein, R. Structure 16, 1407–1416 (2008).
Acknowledgements
We wish to thank J. Dilworth (Sprott Center for Stem Cell Research, Ottawa Hospital Research Institute) for the Ash2L antibody and other reagents. We are highly indebted to R.C. Trievel, who facilitated part of this work. This work was supported by Canadian Institutes of Health Research (CIHR) grants to J.-F.C. (191666) and M.B. (MOP-89834). J.-F.C. holds a Canada Research Chair in Structural Biology and Epigenetics. P.Z. is sponsored by a Natural Sciences and Engineering Research Council of Canada (NSERC) fellowship. C.-P.C. is supported by a CIHR/Thalassemia Foundation of Canada postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
J.-F.C. and M.B. designed the experiments. S.S. and V.A were responsible for doing the EMSA and analyzing the crystal structure. V.T. did the SELEX and validated the ASH2L target genes. C.-P.C. carried out the ChIP and qRT-PCR studies. P.Z. and S.L. did the methyltransferase assays and sequence alignment, respectively. J.S.B. collected the data and calculated the initial phases of the model. A.B. analyzed the SELEX data. J.-F.C. provided scientific direction for the project and wrote the manuscript. J.S.B., A.B. and M.B commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Tables 1 and 2, and Supplementary Methods (PDF 3268 kb)
Rights and permissions
About this article
Cite this article
Sarvan, S., Avdic, V., Tremblay, V. et al. Crystal structure of the trithorax group protein ASH2L reveals a forkhead-like DNA binding domain. Nat Struct Mol Biol 18, 857–859 (2011). https://doi.org/10.1038/nsmb.2093
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2093
This article is cited by
-
lncCPSET1 acts as a scaffold for MLL2/COMPASS to regulate Bmp4 and promote the formation of chicken primordial germ cells
Molecular Genetics and Genomics (2024)
-
DNA binding by PHF1 prolongs PRC2 residence time on chromatin and thereby promotes H3K27 methylation
Nature Structural & Molecular Biology (2017)
-
Hijacked in cancer: the KMT2 (MLL) family of methyltransferases
Nature Reviews Cancer (2015)
-
Trithorax group proteins: switching genes on and keeping them active
Nature Reviews Molecular Cell Biology (2011)