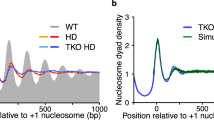
Abstract
A barrier phases nucleosomes at the yeast (Saccharomyces cerevisiae) GAL1–GAL10 genes. Here we separate nucleosome positioning from occupancy and show that the degree of occupancy of these phased sites is predictably determined by the underlying DNA sequences. As this occupancy is increased (by sequence alteration), nucleosome removal upon induction is decreased, as is mRNA production. These results explain why promoter sequences have evolved to form nucleosomes relatively inefficiently.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Floer, M. et al. Cell 141, 407–418 (2010).
Bryant, G.O. et al. PLoS Biol. 6, 2928–2939 (2008).
Chung, H.R. et al. PLoS ONE 5, e15754 (2010).
Locke, G., Tolkunov, D., Moqtaderi, Z., Struhl, K. & Morozov, A.V. Proc. Natl. Acad. Sci. USA 107, 20998–21003 (2010).
Satchwell, S.C., Drew, H.R. & Travers, A.A. J. Mol. Biol. 191, 659–675 (1986).
Segal, E. et al. Nature 442, 772–778 (2006).
Segal, E. & Widom, J. Curr. Opin. Struct. Biol. 19, 65–71 (2009).
Stein, A., Takasuka, T.E. & Collings, C.K. Nucleic Acids Res. 38, 709–719 (2010).
Tillo, D. & Hughes, T.R. BMC Bioinformatics 10, 442 (2009).
Rohs, R. et al. Nature 461, 1248–1253 (2009).
Travers, A.A. & Klug, A. Phil. Trans. R. Soc. Lond. B 317, 537–561 (1987).
Kaplan, N. et al. Nature 458, 362–366 (2009).
Takasuka, T.E. & Stein, A. Nucleic Acids Res. 38, 5672–5680 (2010).
Dechassa, M.L. et al. Mol. Cell 38, 590–602 (2010).
Ahmad, K. & Henikoff, S. Cell 104, 839–847 (2001).
Halpern, M.E. et al. Zebrafish 5, 97–110 (2008).
Mohrmann, L. & Verrijzer, C.P. Biochim. Biophys. Acta 1681, 59–73 (2005).
Wilson, B., Erdjument-Bromage, H., Tempst, P. & Cairns, B.R. Genetics 172, 795–809 (2006).
Tillo, D. et al. PLoS ONE 5, e9129 (2010).
Bernstein, B.E., Liu, C.L., Humphrey, E.L., Perlstein, E.O. & Schreiber, S.L. Genome Biol. 5, R62 (2004).
Lee, C.K., Shibata, Y., Rao, B., Strahl, B.D. & Lieb, J.D. Nat. Genet. 36, 900–905 (2004).
Sekinger, E.A., Moqtaderi, Z. & Struhl, K. Mol. Cell 18, 735–748 (2005).
Zhang, Y. et al. Nat. Struct. Mol. Biol. 16, 847–852 (2009).
Ptashne, M. Curr. Biol. 19, R234–R241 (2009).
Acknowledgements
We thank E. Segal (Weizmann Institute of Science) and J. Widom (Northwestern University) for the “superbinder” sequence and S. Narayan, G. Berrozpe, A. Gann and D. Rhodes for helpful discussions. This work was supported by US National Institutes of Health grant GM032308 to M.P.
Author information
Authors and Affiliations
Contributions
X.W., G.O.B. and M.P. designed the experiments. X.W., G.O.B. and D.S. performed the experiments. X.W. and G.O.B. analyzed the data. X.W., G.O.B., M.F. and M.P. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1, Supplementary Table 1 and Supplementary Methods (PDF 290 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Bryant, G., Floer, M. et al. An effect of DNA sequence on nucleosome occupancy and removal. Nat Struct Mol Biol 18, 507–509 (2011). https://doi.org/10.1038/nsmb.2017
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2017
This article is cited by
-
DNA signals at isoform promoters
Scientific Reports (2016)
-
Nucleosome architecture throughout the cell cycle
Scientific Reports (2016)
-
H2A.Z controls the stability and mobility of nucleosomes to regulate expression of the LH genes
Nature Communications (2016)
-
Principles of a switch
Nature Chemical Biology (2011)