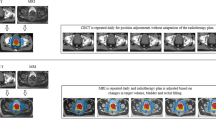
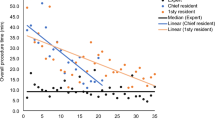
Abstract
Robotic prostatectomy is a common surgical treatment for men with prostate cancer, with some studies estimating that 80% of prostatectomies now performed in the USA are done so robotically. Despite the technical advantages offered by robotic systems, functional and oncological outcomes of prostatectomy can still be improved further. Alternative minimally invasive treatments that have also adopted robotic platforms include brachytherapy and high-intensity focused ultrasonography (HIFU). These techniques require real-time image guidance—such as ultrasonography or MRI—to be truly effective; issues with software compatibility as well as image registration and tracking currently limit such technologies. However, image-guided robotics is a fast-growing area of research that combines the improved ergonomics of robotic systems with the improved visualization of modern imaging modalities. Although the benefits of a real-time image-guided robotic system to improve the precision of surgical interventions are being realized, the clinical usefulness of many of these systems remains to be seen.
Key Points
-
Precise treatment techniques are critical for improving the oncological and functional outcomes of men with prostate cancer; urologists are increasingly performing robot-assisted surgeries, which benefit from reduced tremor and improved field of view
-
Image-guided robotic surgery is expanding in use. Although MRI guidance is more accurate than ultrasonography, it is still operator-dependent and has ergonomic issues owing to the scanner design
-
MRI coupled with transrectal ultrasonography (TRUS) might be a practical alternative to MRI guidance, as it has demonstrated improved sensitivity and specificity over TRUS alone in biopsy and could be applied to robot-assisted brachytherapy and focal ablation
-
Specially designed image-guided robotic platforms for low-dose brachytherapy and prostate biopsy have demonstrated the ability to place brachytherapy seeds accurately and automatically, reducing the exposure of the operator to radiation
-
Initial clinical studies have demonstrated the ability of image guidance during robot-assisted laparoscopic prostatectomy to reduce the rate of positive surgical margins and improve the visualization of key anatomical structures
-
Novel imaging techniques such as fluorescence imaging and ultra-small particles of iron-oxide (USPIO) have opened up the possibility of image-guided targeted robotic lymph node dissection
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Liss, M. A. et al. Robot-assisted radical prostatectomy: 5-year oncological and biochemical outcomes. J. Urol. 188, 2205–2210 (2012).
Illing, R. & Emberton, M. Sonablate-500: transrectal high-intensity focused ultrasound for the treatment of prostate cancer. Expert Rev. Med. Devices 3, 717–729 (2006).
Chaussy, C. G. & Thüroff, S. Robot-assisted high-intensity focused ultrasound in focal therapy of prostate cancer. J. Endourol. 24, 843–847 (2010).
Fichtinger, G. et al. Robotic assistance for ultrasound-guided prostate brachytherapy. Med. Image Anal. 12, 535–545 (2008).
van den Bosch, M. R. et al. MRI-guided robotic system for transperineal prostate interventions: proof of principle. Phys. Med. Biol. 55, N133–140 (2010).
Abdelaziz, S. et al. Design considerations for a novel MRI compatible manipulator for prostate cryoablation. Int. J. Comput. Assist. Radiol. Surg. 6, 811–819 (2011).
Bozzini, G. et al. Focal therapy of prostate cancer: energies and procedures. Urol. Oncol. 31, 155–167 (2013).
Pondman, K. M. et al. MR-guided biopsy of the prostate: an overview of techniques and a systematic review. Eur. Urol. 54, 517–527 (2008).
Brouwer, O. R. et al. SPECT/CT and a portable gamma-camera for image-guided laparoscopic sentinel node biopsy in testicular cancer. J. Nucl. Med. 52, 551–554 (2011).
Yakar, D. et al. Predictive value of MRI in the localization, staging, volume estimation, assessment of aggressiveness, and guidance of radiotherapy and biopsies in prostate cancer. J. Magn. Reson. Imaging 35, 20–31 (2012).
Ho, H., Yuen, J. S., Mohan, P., Lim, E. W. & Cheng, C. W. Robotic transperineal prostate biopsy: pilot clinical study. Urology 78, 1203–1208 (2011).
Ho, H., Yuen, J. S. & Cheng, C. W. Robotic prostate biopsy and its relevance to focal therapy of prostate cancer. Nat. Rev. Urol. 8, 579–585 (2011).
Wei, Z., Ding, M., Downey, D. & Fenster, A. 3D TRUS guided robot assisted prostate brachytherapy. Med. Image Comput. Comput. Assist. Interv. 8, 17–24 (2005).
Long, J.-A. et al. Development of a novel robot for transperineal needle based interventions: focal therapy, brachytherapy and prostate biopsies. J. Urol. 188, 1369–1374 (2012).
Brawer, M. K., Deering, R. E., Brown, M., Preston, S. D. & Bigler, S. A. Predictors of pathologic stage in prostatic carcinoma. The role of neovascularity. Cancer 73, 678–687 (1994).
Aigner, F. et al. Contrast-enhanced ultrasonography using cadence-contrast pulse sequencing technology for targeted biopsy of the prostate. BJU Int. 103, 458–463 (2009).
Halpern, E. J., Gomella, L. G., Forsberg, F., McCue, P. A. & Trabulsi, E. J. Contrast enhanced transrectal ultrasound for the detection of prostate cancer: a randomized, double-blind trial of dutasteride pretreatment. J. Urol. 188, 1739–1745 (2012).
Mitterberger, M. et al. Comparison of contrast enhanced color Doppler targeted biopsy to conventional systematic biopsy: impact on Gleason score. J. Urol. 178, 464–468 (2007).
Frauscher, F. et al. Comparison of contrast enhanced color Doppler targeted biopsy with conventional systematic biopsy: impact on prostate cancer detection. J. Urol. 167, 1648–1652 (2002).
Strazdina, A., Krumina, G. & Sperga, M. The value and limitations of contrast-enhanced ultrasound in detection of prostate cancer. Anticancer Res. 31, 1421–1426 (2011).
Wink, M. et al. Contrast-enhanced ultrasound and prostate cancer; a multicentre European research coordination project. Eur. Urol. 54, 982–992 (2008).
Trabulsi, E. J., Sackett, D., Gomella, L. G. & Halpern, E. J. Enhanced transrectal ultrasound modalities in the diagnosis of prostate cancer. Urology 76, 1025–1033 (2010).
Aigner, F. et al. Value of real-time elastography targeted biopsy for prostate cancer detection in men with prostate specific antigen 1.25 ng/ml or greater and 4.00 ng/ml or less. J. Urol. 184, 913–917 (2010).
Brock, M. et al. The impact of real-time elastography guiding a systematic prostate biopsy to improve cancer detection rate: a prospective study of 353 patients. J. Urol. 187, 2039–2043 (2012).
Sumura, M. et al. Initial evaluation of prostate cancer with real-time elastography based on step-section pathologic analysis after radical prostatectomy: a preliminary study. Int. J. Urol. 14, 811–816 (2007).
Yan, Z. et al. Role of transrectal real-time tissue elastography in the diagnosis of prostate cancer. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 33, 175–179 (2011).
Nelson, E. D., Slotoroff, C. B., Gomella, L. G. & Halpern, E. J. Targeted biopsy of the prostate: the impact of color Doppler imaging and elastography on prostate cancer detection and Gleason score. Urology 70, 1136–1140 (2007).
Pozzi, E. et al. The role of the elastography in the diagnosis of prostate cancer: a retrospective study on 460 patients. Arch. Ital. Urol. Androl. 84, 151–154 (2012).
Kapoor, A., Kapoor, A., Mahajan, G. & Sidhu, B. S. Real-time elastography in the detection of prostate cancer in patients with raised PSA level. Ultrasound Med. Biol. 37, 1374–1381 (2011).
Zhu, Y. et al. Prostate cancer detection with real-time elastography using a bi-plane transducer: comparison with step section radical prostatectomy pathology. World J. Urol. (in press).
Salomon, G. et al. Evaluation of prostate cancer detection with ultrasound real-time elastography: a comparison with step section pathological analysis after radical prostatectomy. Eur. Urol. 54, 1354–1362 (2008).
Brock, M. et al. Multiparametric ultrasound of the prostate: adding contrast enhanced ultrasound to real-time elastography to detect histopathologically confirmed cancer. J. Urol. 189, 93–98 (2013).
Walz, J. et al. Identification of the prostate cancer index lesion by real-time elastography: considerations for focal therapy of prostate cancer. World J. Urol. 29, 589–594 (2011).
Pallwein, L. et al. Real-time elastography for detecting prostate cancer: preliminary experience. BJU Int. 100, 42–46 (2007).
Simmons, L. A. et al. Detection, localisation and characterisation of prostate cancer by prostate HistoScanning(™). BJU Int. 110, 28–35 (2012).
Harvey, C. J., Pilcher, J., Richenberg, J., Patel, U. & Frauscher, F. Applications of transrectal ultrasound in prostate cancer. Br. J. Radiol. 8, S3–S17 (2012).
Beyersdorff, D. et al. Patients with a history of elevated prostate-specific antigen levels and negative transrectal US-guided quadrant or sextant biopsy results: value of MR imaging. Radiology 224, 701–706 (2002).
Riches, S. F. et al. MRI in the detection of prostate cancer: combined apparent diffusion coefficient, metabolite ratio, and vascular parameters. AJR Am. J. Roentgenol. 193, 1583–1591 (2009).
Krieger, A. et al. Design of a novel MRI compatible manipulator for image guided prostate interventions. IEEE Trans. Biomed. Eng. 52, 306–313 (2005).
Krieger, A. et al. An MRI-compatible robotic system with hybrid tracking for MRI-guided prostate intervention. IEEE Trans. Biomed. Eng. 58, 3049–3060 (2011).
Stoianovici, D. et al. “MRI Stealth” robot for prostate interventions. Minim. Invasive Ther. Allied Technol. 16, 241–248 (2007).
Goldenberg, A. A. et al. Robotic system for closed-bore MRI-guided prostatic interventions. IEEE ASME Trans. Mechatron. 13, 374–379 (2008).
Zangos, S. et al. MR-compatible assistance system for biopsy in a high-field-strength system: initial results in patients with suspicious prostate lesions. Radiology 259, 903–910 (2011).
Yakar, D. et al. Feasibility of a pneumatically actuated MR-compatible robot for transrectal prostate biopsy guidance. Radiology 260, 241–247 (2011).
Seifabadi, R. & Cho, N. Accuracy study of a robotic system for MRI-guided prostate needle placement. Int. J. Med. Robot. http://dx.doi.org/10.1002/rcs.1440.
Su, H. et al. High-field MRI-compatible needle placement robot for prostate interventions. Stud. Health Technol. Inform. 163, 623–629 (2011).
Elhawary, H. et al. Robotic system for transrectal biopsy of the prostate: real-time guidance under MRI. IEEE Eng. Med. Biol. Mag. 29, 78–86 (2010).
Delongchamps, N. B. et al. Pre-biopsy Magnetic Resonance Imaging and prostate cancer detection: comparison of random and MRI-targeted biopsies using three different techniques of MRI-TRUS image registration. J. Urol. 189, 493–499 (2012).
Mouraviev, V. et al. The feasibility of multiparametric magnetic resonance imaging for targeted biopsy using novel navigation systems to detect early stage of prostate cancer: the preliminary experience. J. Endourol. http://dx.doi.org/10.1089/end.2012.0215.
Ukimura, O. et al. Technique for a hybrid system of real-time transrectal ultrasound with preoperative magnetic resonance imaging in the guidance of targeted prostate biopsy. Int. J. Urol. 17, 890–893 (2010).
Xu, S. et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput. Aided Surg. 13, 255–264 (2008).
Hu, J. C. et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J. Urol. 175, 541–546 (2006).
Miller, J., Smith, A., Kouba, E., Wallen, E. & Pruthi, R. S. Prospective evaluation of short-term impact and recovery of health related quality of life in men undergoing robotic assisted laparoscopic radical prostatectomy versus open radical prostatectomy. J. Urol. 178, 854–858; discussion 859 (2007).
Tewari, A., Srivasatava, A. & Menon, M. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 92, 205–210 (2003).
Simpfendörfer, T. et al. Augmented reality visualization during laparoscopic radical prostatectomy. J. Endourol. 25, 1841–1845 (2011).
Cohen, D. et al. Augmented reality image guidance in minimally invasive prostatectomy. Lect. Notes Comput. Sci. 6367, 101–110 (2010).
Teber, D. et al. In-vitro evaluation of a soft-tissue navigation system for laparoscopic prostatectomy. J. Endourol. 24, 1487–1491 (2010).
Ukimura, O., Magi-Galluzzi, C. & Gill, I. S. Real-time transrectal ultrasound guidance during laparoscopic radical prostatectomy: impact on surgical margins. J. Urol. 175, 1304–1310 (2006).
Han, M. et al. Tandem-robot assisted laparoscopic radical prostatectomy to improve the neurovascular bundle visualization: a feasibility study. Urology 77, 502–506 (2011).
Hung, A. J. et al. Robotic transrectal ultrasonography during robot-assisted radical prostatectomy. Eur. Urol. 62, 341–348 (2012).
Long, J.-A. et al. Real-time robotic transrectal ultrasound navigation during robotic radical prostatectomy: initial clinical experience. Urology 80, 608–613 (2012).
Van der Poel, H. G., De Blok, W., Bex, A., Meinhardt, W. & Horenblas, S. Peroperative transrectal ultrasonography-guided bladder neck dissection eases the learning of robot-assisted laparoscopic prostatectomy. BJU Int. 102, 849–852 (2008).
Renard-Penna, R. et al. Accuracy of high resolution (1.5 tesla) pelvic phased array magnetic resonance imaging (MRI) in staging prostate cancer in candidates for radical prostatectomy: results from a prospective study. Urol. Oncol. 31, 448–454 (2011).
Bloch, B. N. et al. Prediction of prostate cancer extracapsular extension with high spatial resolution dynamic contrast-enhanced 3-T MRI. Eur. Radiol. 22, 2201–2210 (2012).
Thompson, S. A., Penney, G. P., Hawkes, D. J. & Dasgupta, P. Bone segmentation using a CT based statistical shape model for image guidance in robot assisted prostatectomy [abstract p77]. BJU Int. 101 (Suppl. s5), 40 (2008).
Ukimura, O. & Gill, I. S. Imaging-assisted endoscopic surgery: Cleveland Clinic experience. J. Endourol. 22, 803–810 (2008).
Janetschek, G. Can sentinel pelvic lymph node dissection replace extended pelvic lymph node dissection in patients with prostate cancer? Nat. Clin. Pract Urol. 4, 636–637 (2007).
Khoder, W. Y. et al. Risk factors for pelvic lymphoceles post-radical prostatectomy. Int. J. Urol. 18, 638–643 (2011).
van der Poel, H. G., Tillier, C., De Blok, W. & Van Muilekom, E. Extended nodal dissection reduces sexual function recovery after robot-assisted laparoscopic prostatectomy. J. Endourol. 26, 1192–1198 (2012).
Brouwer, O. R. et al. Image navigation as a means to expand the boundaries of fluorescence-guided surgery. Phys. Med. Biol. 57, 3123–3136 (2012).
Meinhardt, W. et al. Laparoscopic sentinel lymph node biopsy for prostate cancer: the relevance of locations outside the extended dissection area. Prostate Cancer 2012, 751753 (2012).
Harisinghani, M. et al. Noninvasive detection of clinically occult lymph-node metastasesin prostate cancer. N. Engl. J. Med. 348, 2491–2499 (2003).
Heesakkers, R. A. et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 9, 850–856 (2008).
Podder, T., Buzurovic, I., Huang, K. & Yu, Y. MIRAB: an image-guided multichannel robot for prostate brachytherapy. Bodine J. 3, 39 (2010).
Yu, Y. et al. Robotic system for prostate brachytherapy. Comput. Aided Surg. 12, 366–370 (2007).
Salcudean, S. E., Prananta, T. D., Morris, W. J. & Spadinger, I. A robotic needle guide for prostate brachytherapy. IEEE Int. Conf. Robot. Autom. 2975–2981 (2008).
Fichtinger, G. et al. System for robotically assisted prostate biopsy and therapy with intraoperative CT guidance. Acad. Radiol. 9, 60–74 (2002).
Cunha, J. A. et al. Toward adaptive stereotactic robotic brachytherapy for prostate cancer: demonstration of an adaptive workflow incorporating inverse planning and an MR stealth robot. Minim. Invasive Ther. Allied Technol. 19, 189–202 (2011).
Karavitakis, M., Ahmed, H. U., Abel, P. D., Hazell, S. & Winkler, M. H. Tumor focality in prostate cancer: implications for focal therapy. Nat. Rev. Clin. Oncol. 8, 48–55 (2011).
Ahmed, H. U., Arya, M., Freeman, A. & Emberton, M. Do low-grade and low-volume prostate cancers bear the hallmarks of malignancy? Lancet Oncol. 13, e509–e517 (2012).
Jolesz, F. A. MRI-guided focused ultrasound surgery. Ann. Rev. Med. 60, 417–430 (2009).
Hynynen, K. MRIgHIFU: a tool for image-guided therapeutics. J. Magn. Reson. Imaging 34, 482–493 (2011).
Davies, B. L., Harris, S. J. & Dibble, E. Brachytherapy--an example of a urological minimally invasive robotic procedure. Int. J. Med. Robotics Comput. 1, 88–96 (2004).
Schneider, C. M., Okamura, A. M. & Fichtinger, G. A robotic system for transrectal needle insertion into the prostate with integrated ultrasound. IEEE Int. Conf. Robot. Autom. 1, 365–370 (2004).
Phee, L. et al. Ultrasound guided robotic system for transperineal biopsy of the prostate. IEEE Int. Conf. Robot. Autom. 1315–1320 (2005).
Bassan, H. S., Patel, R. V. & Moallem, M. A novel manipulator for percutaneous needle insertion: design and experimentation. IEEE ASME Trans. Mechatron. 14, 746–761 (2009).
Muntener, M. et al. Transperineal prostate intervention: robot for fully automated MR imaging--system description and proof of principle in a canine model. Radiology 247, 543–549 (2008).
DiMaio, S. P. et al. Robot-assisted needle placement in open MRI: system architecture, integration and validation. Comput. Aided Surg. 12, 15–24 (2007).
Fischer, G. S. et al. MRI-compatible pneumatic robot for transperineal prostate needle placement. IEEE ASME Trans. Mechatron. 13, 295–305 (2008).
Su, H. et al. A MRI-guided concentric tube continuum robot with piezoelectric actuation: a feasibility study. IEEE Int. Conf. Robot. Autom. 1939–1945 (2012).
Fitzpatrick, J. M., Hill, D. L. & Maurer, C. R. Jr in Handbook of Medical Imaging, Volume 2. Medical Image Processing and Analysis (eds Fitzpatrick, J. M. & Sonka, M.) 447–513 (SPIE Press Monograph, 2000).
Author information
Authors and Affiliations
Contributions
A. N. Sridhar and A. Hughes-Hallett contributed equally to this manuscript. A. N. Sridhar and A. Hughes-Hallett researched the data for the article. A. N. Sridhar, A. Hughes-Hallett, E. K. Mayer and P. J. Pratt discussed the article's content, after which A. N. Sridhar, A. Hughes-Hallett and E. K. Mayer wrote the manuscript. All authors edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sridhar, A., Hughes-Hallett, A., Mayer, E. et al. Image-guided robotic interventions for prostate cancer. Nat Rev Urol 10, 452–462 (2013). https://doi.org/10.1038/nrurol.2013.129
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2013.129
This article is cited by
-
Arc-to-line frame registration method for ultrasound and photoacoustic image-guided intraoperative robot-assisted laparoscopic prostatectomy
International Journal of Computer Assisted Radiology and Surgery (2023)
-
Techniques and Outcomes of MRI-TRUS Fusion Prostate Biopsy
Current Urology Reports (2021)
-
Surgical navigation system for laparoscopic lateral pelvic lymph node dissection in rectal cancer surgery using laparoscopic-vision-tracked ultrasonic imaging
Surgical Endoscopy (2021)
-
Personalized heterogeneous deformable model for fast volumetric registration
BioMedical Engineering OnLine (2017)
-
Looking beyond the imaging plane: 3D needle tracking with a linear array ultrasound probe
Scientific Reports (2017)