Abstract
Erectile dysfunction is a prevalent condition that leads to significant morbidity and distress, not just for affected men but also for their partners. Very few currently available treatments ameliorate the underlying causes of the disorder and 'cure' the disease state. Much recent effort has been focused on the development of gene and cell-based approaches to rectify the molecular and tissue defects responsible for ED. Gene therapy has been investigated in animal models as a means to restore normal function to the penis; at this time, however, only one human trial has been published in the peer-reviewed literature. Recent gene therapy studies have focused on the modulation of enzymes associated with the NOS/cGMP pathway, and supplementation of trophic factors, peptides and potassium channels. Stem cell therapy has been a topic of interest in more recent years but there are currently very few published reports in animal models and none in human men. Although stem cell therapy offers the potential for restoration of functional tissues, legitimate concerns remain regarding the long-term fate of stem cells. The long-term safety of both gene and stem cell therapy must be thoroughly investigated before large-scale human studies can be considered.
Key Points
-
Gene and stem cell-based therapies for the treatment of erectile dysfunction offer the potential to rejuvenate tissues that have been damaged by a variety of physiological insults
-
A number of viral vectors for gene delivery are available, each with their own merits and drawbacks
-
Modulation of the NOS/cGMP pathway and supplementation of trophic factors have been the principle foci of gene therapy investigations, although a number of other treatment approaches have also been considered
-
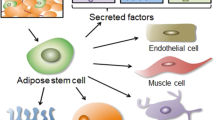
Stem cell therapy offers the potential for restoration of functional tissues via differentiation of stem cells or possibly by release of trophic factors
-
Despite the potential therapeutic benefits, stem cell therapy is currently limited by ethical issues, risk of disease, and host morbidity
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Laumann, E. O. et al. Prevalence and correlates of erectile dysfunction by race and ethnicity among men aged 40 or older in the United States: from the male attitudes regarding sexual health survey. J. Sex. Med. 4, 57–65 (2007).
Prins, J., Blanker, M. H., Bohnen, A. M., Thomas, S. & Bosch, J. L. Prevalence of erectile dysfunction: a systematic review of population-based studies. Int. J. Impot. Res. 14, 422–432 (2002).
Andersson, K. E. & Wagner, G. Physiology of penile erection. Physiol. Rev. 75, 191–236 (1995).
Padma-Nathan, H. et al. Pharmacotherapy for erectile dysfunction. J. Sex. Med. 1, 128–140 (2004).
Gonzalez-Cadavid, N. F., Ignarro, L. J. & Rajfer, J. Nitric oxide and the cyclic GMP system in the penis. Mol. Urol. 3, 51–59 (1999).
Russell, S. & McVary, K. T. Lower urinary tract symptoms and erectile dysfunction: epidemiology and treatment in the aging man. Curr. Urol. Rep. 6, 445–453 (2005).
Saenz de Tejada, I. et al. Pathophysiology of erectile dysfunction. J. Sex. Med. 2, 26–39 (2005).
Azadzoi, K. M., Schulman, R. N., Aviram, M. & Siroky, M. B. Oxidative stress in arteriogenic erectile dysfunction: prophylactic role of antioxidants. J. Urol. 174, 386–393 (2005).
Jones, R. W. et al. Oxygen free radicals and the penis. Expert Opin. Pharmacother. 3, 889–897 (2002).
Kim, J. A., Montagnani, M., Koh, K. K. & Quon, M. J. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113, 1888–1904 (2006).
Saenz de Tejada, I., Goldstein, I., Azadzoi, K., Krane, R. J. & Cohen, R. A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N. Engl. J. Med. 320, 1025–1030 (1989).
Cartledge, J. J., Eardley, I. & Morrison, J. F. Impairment of corpus cavernosal smooth muscle relaxation by glycosylated human haemoglobin. BJU Int. 85, 735–741 (2000).
Azadzoi, K. M. & Saenz de Tejada, I. Hypercholesterolemia impairs endothelium-dependent relaxation of rabbit corpus cavernosum smooth muscle. J. Urol. 146, 238–240 (1991).
Kato, R. et al. Herpes simplex virus vector-mediated delivery of glial cell line-derived neurotrophic factor rescues erectile dysfunction following cavernous nerve injury. Gene Ther. 14, 1344–1352 (2007).
Somia, N. & Verma, I. M. Gene therapy: trials and tribulations. Nat. Rev. Genet. 1, 91–99 (2000).
Temin, H. M. Safety considerations in somatic gene therapy of human disease with retrovirus vectors. Hum. Gene Ther. 1, 111–123 (1990).
Brenner, M. Gene transfer by adenovectors. Blood 94, 3965–3967 (1999).
Samaniego, L. A., Webb, A. L. & DeLuca, N. A. Functional interactions between herpes simplex virus immediate-early proteins during infection: gene expression as a consequence of ICP27 and different domains of ICP4. J. Virol. 69, 5705–5715 (1995).
Abdel Aziz, M. T. et al. Effect of HO-1 cDNA-liposome complex transfer on erectile signalling of aged rats. Andrologia 41, 176–183 (2009).
Chancellor, M. B. et al. Nitric oxide synthase gene transfer for erectile dysfunction in a rat model. BJU Int. 91, 691–696 (2003).
Deng, W. et al. Adenoviral gene transfer of eNOS: high-level expression in ex vivo expanded marrow stromal cells. Am. J. Physiol. Cell Physiol. 285, C1322–C1329 (2003).
Dall'Era, J. E. et al. Vascular endothelial growth factor (VEGF) gene therapy using a nonviral gene delivery system improves erectile function in a diabetic rat model. Int. J. Impot. Res. 20, 307–314 (2008).
Kichler, A. Gene transfer with modified polyethylenimines. J. Gene Med. 6 (Suppl. 1), S3–S10 (2004).
Bivalacqua, T. J. & Hellstrom, W. J. Potential application of gene therapy for the treatment of erectile dysfunction. J. Androl. 22, 183–190 (2001).
Christ, G. J. & Melman, A. The application of gene therapy to the treatment of erectile dysfunction. Int. J. Impot. Res. 10, 111–112 (1998).
Melman, A. & Christ, G. J. Integrative erectile biology. The effects of age and disease on gap junctions and ion channels and their potential value to the treatment of erectile dysfunction. Urol. Clin. North Am. 28, 217–231 (2001).
Christ, G. J., Brink, P. R., Melman, A. & Spray, D. C. The role of gap junctions and ion channels in the modulation of electrical and chemical signals in human corpus cavernosum smooth muscle. Int. J. Impot. Res. 5, 77–96 (1993).
Griffith, O. W. & Stuehr, D. J. Nitric oxide synthases: properties and catalytic mechanism. Annu. Rev. Physiol. 57, 707–736 (1995).
Burnett, A. L. et al. Nitric oxide-dependent penile erection in mice lacking neuronal nitric oxide synthase. Mol. Med. 2, 288–296 (1996).
Hedlund, P. et al. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc. Natl Acad. Sci. USA 97, 2349–2354 (2000).
Garban, H. et al. Cloning of rat and human inducible penile nitric oxide synthase. Application for gene therapy of erectile dysfunction. Biol. Reprod. 56, 954–963 (1997).
Bivalacqua, T. J. et al. Gene transfer of endothelial nitric oxide synthase partially restores nitric oxide synthesis and erectile function in streptozotocin diabetic rats. J. Urol. 169, 1911–1917 (2003).
Burnett, A. L. Role of nitric oxide in the physiology of erection. Biol. Reprod. 52, 485–489 (1995).
Bivalacqua, T. J. et al. A rat model of Peyronie's disease associated with a decrease in erectile activity and an increase in inducible nitric oxide synthase protein expression. J. Urol. 163, 1992–1998 (2000).
Hung., A. et al. Expression of inducible nitric oxide synthase in smooth muscle cells from rat penile corpora cavernosa. J. Androl. 16, 469–481 (1995).
Bivalacqua, T. J., Deng, W., Champion, H. C., Hellstrom, W. J. & Kadowitz, P. J. Gene therapy techniques for the delivery of endothelial nitric oxide synthase to the corpora cavernosa for erectile dysfunction. Methods Mol. Biol. 279, 173–185 (2004).
Bivalacqua, T. J. et al. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am. J. Physiol. Heart Circ. Physiol. 292, H1278–H1290 (2007).
Hofmann, F., Feil, R., Kleppisch, T. & Schlossmann, J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol. Rev. 86, 1–23 (2006).
Chang, S. et al. Downregulation of cGMP-dependent protein kinase-1 activity in the corpus cavernosum smooth muscle of diabetic rabbits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R950–R960 (2004).
Bivalacqua, T. J. et al. Dysregulation of cGMP-dependent protein kinase 1 (PKG-1) impairs erectile function in diabetic rats: influence of in vivo gene therapy of PKG1alpha. BJU Int. 99, 1488–1494 (2007).
Liu, Y. et al. Carbon monoxide and nitric oxide suppress the hypoxic induction of vascular endothelial growth factor gene via the 5′ enhancer. J. Biol. Chem. 273, 15257–15262 (1998).
Abraham, N. G. & Kappas, A. Heme oxygenase and the cardiovascular-renal system. Free Radic. Biol. Med. 39, 1–25 (2005).
Aydin, A. et al. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: effects of glycemic control. Clin. Biochem. 34, 65–70 (2001).
Cellek, S., Foxwell, N. A. & Moncada, S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes 52, 2353–2362 (2003).
Ryu, J. K. et al. The role of free radical in the pathogenesis of impotence in streptozotocin-induced diabetic rats. Yonsei Med. J. 44, 236–241 (2003).
Bivalacqua, T. J. et al. Superoxide anion production in the rat penis impairs erectile function in diabetes: influence of in vivo extracellular superoxide dismutase gene therapy. J. Sex. Med. 2, 187–197 (2005).
Jaffrey, S. R. & Snyder, S. H. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science 274, 774–777 (1996).
Good, L. Translation repression by antisense sequences. Cell. Mol. Life Sci. 60, 854–861 (2003).
Magee, T. R. et al. Protein inhibitor of nitric oxide synthase (NOS) and the N-methyl-D-aspartate receptor are expressed in the rat and mouse penile nerves and colocalize with penile neuronal NOS. Biol. Reprod. 68, 478–488 (2003).
Magee, T. R. et al. Antisense and short hairpin RNA (shRNA) constructs targeting PIN (Protein Inhibitor of NOS) ameliorate aging-related erectile dysfunction in the rat. J. Sex. Med. 4, 633–643 (2007).
Mori, M. & Gotoh, T. Regulation of nitric oxide production by arginine metabolic enzymes. Biochem. Biophys. Res. Commun. 275, 715–719 (2000).
Bivalacqua, T. J., Hellstrom, W. J., Kadowitz, P. J. & Champion, H. C. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem. Biophys. Res. Commun. 283, 923–927 (2001).
Kim, N. N. et al. Probing erectile function: S-(2-boronoethyl)-L-cysteine binds to arginase as a transition state analogue and enhances smooth muscle relaxation in human penile corpus cavernosum. Biochemistry 40, 2678–2688 (2001).
Bivalacqua, T. J., Burnett, A. L., Hellstrom, W. J. & Champion, H. C. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am. J. Physiol. Heart Circ. Physiol. 292, H1340–H1351 (2007).
Chang, S. et al. Increased contractility of diabetic rabbit corpora smooth muscle in response to endothelin is mediated via Rho-kinase beta. Int. J. Impot. Res. 15, 53–62 (2003).
Bivalacqua, T. J. et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc. Natl Acad. Sci. USA 101, 9121–9126 (2004).
Rogers, R. S., Graziottin, T. M., Lin, C. S., Kan, Y. W. & Lue, T. F. Intracavernosal vascular endothelial growth factor (VEGF) injection and adeno-associated virus-mediated VEGF gene therapy prevent and reverse venogenic erectile dysfunction in rats. Int. J. Impot. Res. 15, 26–37 (2003).
Markus, A., Patel, T. D. & Snider, W. D. Neurotrophic factors and axonal growth. Curr. Opin. Neurobiol. 12, 523–531 (2002).
Laurikainen, A., Hiltunen, J. O., Vanhatalo, S., Klinge, E. & Saarma, M. Glial cell line-derived neurotrophic factor is expressed in penis of adult rat and retrogradely transported in penile parasympathetic and sensory nerves. Cell Tissue Res. 302, 321–329 (2000).
Palma, C. A. & Keast, J. R. Structural effects and potential changes in growth factor signalling in penis-projecting autonomic neurons after axotomy. BMC Neurosci. 7, 41 (2006).
Wanigasekara, Y., Airaksinen, M. S., Heuckeroth, R. O., Milbrandt, J. & Keast, J. R. Neurturin signalling via GFRalpha2 is essential for innervation of glandular but not muscle targets of sacral parasympathetic ganglion neurons. Mol. Cell Neurosci. 25, 288–300 (2004).
Wanigasekara, Y. & Keast, J. R. Neurturin has multiple neurotrophic effects on adult rat sacral parasympathetic ganglion neurons. Eur. J. Neurosci. 22, 595–604 (2005).
Kato, R. et al. Herpes simplex virus vector-mediated delivery of neurturin rescues erectile dysfunction of cavernous nerve injury. Gene Ther. 16, 26–33 (2009).
Apfel, S. C. Neurotrophic factors in the therapy of diabetic neuropathy. Am. J. Med. 107, 34S–42S (1999).
Lin, G. et al. Neurotrophic effects of vascular endothelial growth factor and neurotrophins on cultured major pelvic ganglia. BJU Int. 92, 631–635 (2003).
Bennet, N. E. et al. Improvement in erectile dysfunction after neurotrophic factor gene therapy in diabetic rats. J. Urol. 173, 1820–1824 (2005).
Bella, A. J. et al. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part, I. J. Sex. Med. 3, 815–820 (2006).
Bakircioglu, M. E. et al. The effect of adeno-associated virus mediated brain derived neurotrophic factor in an animal model of neurogenic impotence. J. Urol. 165, 2103–2109 (2001).
Gholami, S. S. et al. The effect of vascular endothelial growth factor and adeno-associated virus mediated brain derived neurotrophic factor on neurogenic and vasculogenic erectile dysfunction induced by hyperlipidemia. J. Urol. 169, 1577–1581 (2003).
Jung, G. W., Kwak, J. Y., Yoon, S., Yoon, J. H. & Lue, T. F. IGF-I and TGF-beta2 have a key role on regeneration of nitric oxide synthase (NOS)-containing nerves after cavernous neurotomy in rats. Int. J. Impot. Res. 11, 247–259 (1999).
Bochinski, D. et al. Effect of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 complex in cavernous nerve cryoablation. Int. J. Impot. Res. 16, 418–423 (2004).
El-Sakka, A. I., Lin, C. S., Chui, R. M., Dahiya, R. & Lue, T. F. Effects of diabetes on nitric oxide synthase and growth factor genes and protein expression in an animal model. Int. J. Impot. Res. 11, 123–132 (1999).
Pu, X. Y., Hu, L. Q., Wang, H. P., Luo, Y. X. & Wang, X. H. Improvement in erectile dysfunction after insulin-like growth factor-1 gene therapy in diabetic rats. Asian J. Androl. 9, 83–91 (2007).
Yamanaka, M. et al. Vascular endothelial growth factor restores erectile function through inhibition of apoptosis in diabetic rat penile crura. J. Urol. 173, 318–323 (2005).
Gerber, H. P., Dixit, V. & Ferrara, N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J. Biol. Chem. 273, 13313–13316 (1998).
Ryu, J. K. et al. Combined angiopoietin-1 and vascular endothelial growth factor gene transfer restores cavernous angiogenesis and erectile function in a rat model of hypercholesterolemia. Mol. Ther. 13, 705–715 (2006).
Miller, M. A., Morgan, R. J., Thompson, C. S., Mikhailidis, D. P. & Jeremy, J. Y. Effects of papaverine and vasointestinal polypeptide on penile and vascular cAMP and cGMP in control and diabetic animals: an in vitro study. Int. J. Impot. Res. 7, 91–100 (1995).
Ding, Y. Q., Takada, M., Kaneko, T. & Mizuno, N. Colocalization of vasoactive intestinal polypeptide and nitric oxide in penis-innervating neurons in the major pelvic ganglion of the rat. Neurosci. Res. 22, 129–131 (1995).
Ehmke, H., Junemann, K. P., Mayer, B. & Kummer, W. Nitric oxide synthase and vasoactive intestinal polypeptide colocalization in neurons innervating the human penile circulation. Int. J. Impot. Res. 7, 147–156 (1995).
Shen, Z. J. et al. Gene transfer of vasoactive intestinal polypeptide into the penis improves erectile response in the diabetic rat. BJU Int. 95, 890–894 (2005).
Stief, C. G. et al. Calcitonin gene-related peptide: possibly neurotransmitter contributes to penile erection in monkeys. Urology 41, 397–401 (1993).
Djamilian, M., Stief, C. G., Kuczyk, M. & Jonas, U. Followup results of a combination of calcitonin gene-related peptide and prostaglandin E1 in the treatment of erectile dysfunction. J. Urol. 149, 1296–1298 (1993).
Bivalacqua, T. J., Champion, H. C., Abdel-Mageed, A. B., Kadowitz, P. J. & Hellstrom, W. J. Gene transfer of prepro-calcitonin gene-related peptide restores erectile function in the aged rat. Biol. Reprod. 65, 1371–1377 (2001).
Christ, G. J. The penis as a vascular organ. The importance of corporal smooth muscle tone in the control of erection. Urol. Clin. North Am. 22, 727–745 (1995).
Venkateswarlu, K. et al. Potassium channels and human corporeal smooth muscle cell tone: diabetes and relaxation of human corpus cavernosum smooth muscle by adenosine triphosphate sensitive potassium channel openers. J. Urol. 168, 355–361 (2002).
So, I., Chae, M. R. & Lee, S. W. Gene transfer of the K(ATP) channel restores age-related erectile dysfunction in rats. BJU Int. 100, 1154–1160 (2007).
Robertson, B. E., Schubert, R., Hescheler, J. & Nelson, M. T. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am. J. Physiol. 265, C299–C303 (1993).
Alioua, A. et al. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J. Biol. Chem. 273, 32950–32956 (1998).
Spektor, M. et al. Potassium channels and human corporeal smooth muscle cell tone: further evidence of the physiological relevance of the Maxi-K channel subtype to the regulation of human corporeal smooth muscle tone in vitro. J. Urol. 167, 2628–2635 (2002).
Werner, M. E., Meredith, A. L., Aldrich, R. W. & Nelson, M. T. Hypercontractility and impaired sildenafil relaxations in the BKCa channel deletion model of erectile dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R181–R188 (2008).
Christ, G. J. et al. Intracorporal injection of hSlo cDNA in rats produces physiologically relevant alterations in penile function. Am. J. Physiol. 275, H600–H608 (1998).
Melman, A., Zhao, W., Davies, K. P., Bakal, R. & Christ, G. J. The successful long-term treatment of age related erectile dysfunction with hSlo cDNA in rats in vivo. J. Urol. 170, 285–290 (2003).
Christ, G. J. et al. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am. J. Physiol. Heart Circ. Physiol. 287, H1544–H1553 (2004).
Melman, A., Bar-Chama, N., McCullough, A., Davies, K. & Christ, G. hMaxi-K gene transfer in males with erectile dysfunction: results of the first human trial. Hum. Gene Ther. 17, 1165–1176 (2006).
Bochinski, D. et al. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 94, 904–909 (2004).
Song, Y. et al. Transdifferentiation of rat fetal brain stem cells into penile smooth muscle cells. BJU Int. 104, 257–262 (2009).
Song, Y. S. et al. Human neural crest stem cells transplanted in rat penile corpus cavernosum to repair erectile dysfunction. BJU Int. 102, 220–224 (2008).
Song, Y. S. et al. Potential differentiation of human mesenchymal stem cell transplanted in rat corpus cavernosum toward endothelial or smooth muscle cells. Int. J. Impot.Res. 19, 378–385 (2007).
Nolazco, G. et al. Effect of muscle-derived stem cells on the restoration of corpora cavernosa smooth muscle and erectile function in the aged rat. BJU Int. 101, 1156–1164 (2008).
Lin, G. et al. Potential of adipose-derived stem cells for treatment of erectile dysfunction. J. Sex.Med. 6 (Suppl. 3), 320–327 (2009).
Garcia, M. M. et al. Treatment of erectile dysfunction in the obese type-II diabetic ZDF rat with adipose tissue derived stem cells. J. Sex. Med. (in press).
Huang, Y. C. et al. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J. Sex. Med. (in press).
Smith, L. & Byers, J. F. Gene therapy in the post-Gelsinger era. JONAS Healthc. Law Ethics Regul. 4, 104–110 (2002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
T. F. Lue declares that he has acted as a consultant for Auxilium, Bayer, Eli Lilly, Medtronic and Pfizer, has received grant/research support from American Medical Systems and has been a board member for Genix Healthcare. A. W. Shindel declares that he has acted as a consultant for Boehringer Ingelheim and a section editor for Elsevier Yearbook of Urology. A. Harraz declares no competing interests.
Rights and permissions
About this article
Cite this article
Harraz, A., Shindel, A. & Lue, T. Emerging gene and stem cell therapies for the treatment of erectile dysfunction. Nat Rev Urol 7, 143–152 (2010). https://doi.org/10.1038/nrurol.2010.8
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2010.8
This article is cited by
-
Emerging tools for erectile dysfunction: a role for regenerative medicine
Nature Reviews Urology (2012)
-
Emerging and Novel Therapeutic Approaches in the Treatment of Male Erectile Dysfunction
Current Urology Reports (2011)