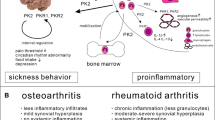
Key Points
-
Microenvironment proteinase-mediated signalling can have a key role in arthritis
-
Proteinase-mediated activation or silencing of proteinase-activated receptors (PARs), cross-activation of transient receptor potential cation channels and release of complement receptor ligands can regulate pain and inflammation in the joint
-
Proteinases and their receptors, including the PARs, represent promising targets for the treatment of arthritic pain and inflammation
-
Either enzyme-selective or broad-spectrum proteinase inhibitors administered in the restricted environment of the joint space over a programmed time frame could prove of value in treating arthritis
Abstract
Proteinases are enzymes with established roles in physiological and pathological processes such as digestion and the homeostasis, destruction and repair of tissues. Over the past few years, the hormone-like properties of circulating proteinases have become increasingly appreciated. Some proteolytic enzymes trigger cell signalling via proteinase-activated receptors, a family of G protein-coupled receptors that have been implicated in inflammation and pain in inflammatory arthritis. Proteinases can also regulate ion flux owing to the cross-sensitization of transient receptor potential cation channel subfamily V members 1 and 4, which are associated with mechanosensing and pain. In this Review, the idea that proteinases have the potential to orchestrate inflammatory signals by interacting with receptors on cells within the synovial microenvironment of an inflamed joint is revisited in three arthritic diseases: osteoarthritis, spondyloarthritis and rheumatoid arthritis. Unanswered questions are highlighted and the therapeutic potential of modulating this proteinase–receptor axis for the management of disease in patients with these types of arthritis is also discussed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Firestein, G. S. & McInnes, I. B. Immunopathogenesis of rheumatoid arthritis. Immunity 46, 183–196 (2017).
Ambarus, C., Yeremenko, N., Tak, P. P. & Baeten, D. Pathogenesis of spondyloarthritis: autoimmune or autoinflammatory? Curr. Opin. Rheumatol 24, 351–358 (2012).
Haseeb, A. & Haqqi, T. M. Immunopathogenesis of osteoarthritis. Clin. Immunol. 146, 185–196 (2013).
Loeser, R. F., Goldring, S. R., Scanzello, C. R. & Goldring, M. B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64, 1697–1707 (2012).
Robinson, W. H. et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 12, 580–592 (2016).
Lubberts, E. The IL-23−IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol 11, 415–429 (2015).
Ackermann, C. & Kavanaugh, A. Tumor necrosis factor as a therapeutic target of rheumatologic disease. Expert Opin. Ther. Targets 11, 1369–1384 (2007).
Noack, M. & Miossec, P. Selected cytokine pathways in rheumatoid arthritis. Semin. Immunopathol. 39, 365–383 (2017).
Glintborg, B. et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum. 63, 382–390 (2011).
Glintborg, B. et al. Clinical response, drug survival and predictors thereof in 432 ankylosing spondylitis patients after switching tumour necrosis factor α inhibitor therapy: results from the Danish nationwide DANBIO registry. Ann. Rheum. Dis. 72, 1149–1155 (2013).
Hetland, M. L. et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 62, 22–32 (2010).
Hermann, W., Lambova, S. & Muller-Ladner, U. Current treatment options for osteoarthritis. Curr. Rheumatol Rev. https://doi.org/10.2174/1573397113666170829155149 (2017).
Rengel, Y., Ospelt, C. & Gay, S. Proteinases in the joint: clinical relevance of proteinases in joint destruction. Arthritis Res. Ther. 9, 221 (2007).
Martel-Pelletier, J., Welsch, D. J. & Pelletier, J. P. Metalloproteases and inhibitors in arthritic diseases. Best Pract. Res. Clin. Rheumatol. 15, 805–829 (2001).
Burrage, P. S., Mix, K. S. & Brinckerhoff, C. E. Matrix metalloproteinases: role in arthritis. Front. Biosci. 11, 529–543 (2006).
Amar, S., Smith, L. & Fields, G. B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta 1864, 1940–1951 (2017).
Muller-Ladner, U., Gay, R. E. & Gay, S. Cysteine proteinases in arthritis and inflammation. Perspect. Drug Discov. 6, 87–98 (1996).
Delaisse, J. M. et al. Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities. Microsc. Res. Tech. 61, 504–513 (2003).
Hou, W. S. et al. Cathepsin K is a critical protease in synovial fibroblast-mediated collagen degradation. Am. J. Pathol. 159, 2167–2177 (2001).
Huet, G. et al. Stimulation of the secretion of latent cysteine proteinase activity by tumor necrosis factor α and interleukin-1. Arthritis Rheum. 36, 772–780 (1993).
Lemaire, R. et al. Selective induction of the secretion of cathepsins B and L by cytokines in synovial fibroblast-like cells. Br. J. Rheumatol. 36, 735–743 (1997).
Kaneko, M. et al. Expression of proteinases and inflammatory cytokines in subchondral bone regions in the destructive joint of rheumatoid arthritis. Rheumatology (Oxford) 40, 247–255 (2001).
Trabandt, A., Gay, R. E., Fassbender, H. G. & Gay, S. Cathepsin B in synovial cells at the site of joint destruction in rheumatoid arthritis. Arthritis Rheum. 34, 1444–1451 (1991).
Cunnane, G. et al. Synovial tissue protease gene expression and joint erosions in early rheumatoid arthritis. Arthritis Rheum. 44, 1744–1753 (2001).
Fiedorczyk, M., Klimiuk, P. A., Sierakowski, S., Gindzienska-Sieskiewicz, E. & Chwiecko, J. Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with early rheumatoid arthritis. J. Rheumatol. 33, 1523–1529 (2006).
Litinsky, I. et al. The effects of leflunomide on clinical parameters and serum levels of IL-6, IL-10, MMP-1 and MMP-3 in patients with resistant rheumatoid arthritis. Cytokine 33, 106–110 (2006).
Catrina, A. I. et al. Anti-tumour necrosis factor (TNF)-α therapy (etanercept) down-regulates serum matrix metalloproteinase (MMP)-3 and MMP-1 in rheumatoid arthritis. Rheumatology (Oxford) 41, 484–489 (2002).
Smith, G. N. Jr The role of collagenolytic matrix metalloproteinases in the loss of articular cartilage in osteoarthritis. Front. Biosci. 11, 3081–3095 (2006).
Salminen-Mankonen, H. J., Morko, J. & Vuorio, E. Role of cathepsin K in normal joints and in the development of arthritis. Curr. Drug Targets 8, 315–323 (2007).
Moz, S. et al. Spondyloarthritis: matrix metalloproteinasesas biomarkers of pathogenesis and response to tumor necrosis factor (TNF) inhibitors. Int. J. Mol. Sci. 18, E830 (2017).
Vandooren, B. et al. Involvement of matrix metalloproteinases and their inhibitors in peripheral synovitis and down-regulation by tumor necrosis factor α blockade in spondylarthropathy. Arthritis Rheum. 50, 2942–2953 (2004).
Cretu, D. et al. Identification of psoriatic arthritis mediators in synovial fluid by quantitative mass spectrometry. Clin. Proteom. 11, 27 (2014).
Jadon, D. R. et al. Serum bone-turnover biomarkers are associated with the occurrence of peripheral and axial arthritis in psoriatic disease: a prospective cross-sectional comparative study. Arthritis Res. Ther. 19, 210 (2017).
Sun, S. et al. The active form of MMP-3 is a marker of synovial inflammation and cartilage turnover in inflammatory joint diseases. BMC Musculoskelet. Disord. 15, 93 (2014).
Ricklin, D., Hajishengallis, G., Yang, K. & Lambris, J. D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 (2010).
Trouw, L. A., Pickering, M. C. & Blom, A. M. The complement system as a potential therapeutic target in rheumatic disease. Nat. Rev. Rheumatol. 13, 538–547 (2017).
Oikonomopoulou, K. et al. Induction of complement C3a receptor responses by kallikrein-related peptidase 14. J. Immunol. 191, 3858–3866 (2013).
Morgan, B. P., Daniels, R. H. & Williams, B. D. Measurement of terminal complement complexes in rheumatoid arthritis. Clin. Exp. Immunol. 73, 473–478 (1988).
Struglics, A. et al. The complement system is activated in synovial fluid from subjects with knee injury and from patients with osteoarthritis. Arthritis Res. Ther. 18, 223 (2016).
Happonen, K. E. et al. Regulation of complement by cartilage oligomeric matrix protein allows for a novel molecular diagnostic principle in rheumatoid arthritis. Arthritis Rheum. 62, 3574–3583 (2010).
Happonen, K. E., Heinegard, D., Saxne, T. & Blom, A. M. Interactions of the complement system with molecules of extracellular matrix: relevance for joint diseases. Immunobiology 217, 1088–1096 (2012).
Neumann, E. et al. Local production of complement proteins in rheumatoid arthritis synovium. Arthritis Rheum. 46, 934–945 (2002).
Gulati, P., Guc, D., Lemercier, C., Lappin, D. & Whaley, K. Expression of the components and regulatory proteins of the classical pathway of complement in normal and diseased synovium. Rheumatol. Int. 14, 13–19 (1994).
Bradley, K. et al. Synthesis of classical pathway complement components by chondrocytes. Immunology 88, 648–656 (1996).
Nakagawa, K. et al. Complement C1s activation in degenerating articular cartilage of rheumatoid arthritis patients: immunohistochemical studies with an active form specific antibody. Ann. Rheum. Dis. 58, 175–181 (1999).
Happonen, K. E. et al. Serum COMP-C3b complexes in rheumatic diseases and relation to anti-TNF-α treatment. Arthritis Res. Ther. 14, R15 (2012).
Liszewski, M. K. et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 39, 1143–1157 (2013).
So, A. K. et al. Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J. Thromb. Haemost. 1, 2510–2515 (2003).
Busso, N. & Hamilton, J. A. Extravascular coagulation and the plasminogen activator/plasmin system in rheumatoid arthritis. Arthritis Rheum. 46, 2268–2279 (2002).
Martel-Pelletier, J. et al. Plasmin, plasminogen activators and inhibitor in human osteoarthritic cartilage. J. Rheumatol. 18, 1863–1871 (1991).
Hoppe, B. & Dorner, T. Coagulation and the fibrin network in rheumatic disease: a role beyond haemostasis. Nat. Rev. Rheumatol. 8, 738–746 (2012).
Amara, U. et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 185, 5628–5636 (2010).
Varisco, P. A. et al. Effect of thrombin inhibition on synovial inflammation in antigen induced arthritis. Ann. Rheum. Dis. 59, 781–787 (2000).
Marty, I. et al. Amelioration of collagen-induced arthritis by thrombin inhibition. J. Clin. Invest. 107, 631–640 (2001).
Busso, N., Morard, C., Salvi, R., Peclat, V. & So, A. Role of the tissue factor pathway in synovial inflammation. Arthritis Rheum. 48, 651–659 (2003).
Nakano, S., Ikata, T., Kinoshita, I., Kanematsu, J. & Yasuoka, S. Characteristics of the protease activity in synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Clin. Exp. Rheumatol. 17, 161–170 (1999).
Mihara, K. et al. Thrombin-mediated direct activation of proteinase-activated receptor-2: another target for thrombin signaling. Mol. Pharmacol. 89, 606–614 (2016).
Hollenberg, M. D. & Compton, S. J. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol. Rev. 54, 203–217 (2002).
Chou, P. Y., Su, C. M., Huang, C. Y. & Tang, C. H. The characteristics of thrombin in osteoarthritic pathogenesis and treatment. Biomed. Res. Int. 2014, 407518 (2014).
Eissa, A. et al. Serum kallikrein-8 correlates with skin activity, but not psoriatic arthritis, in patients with psoriatic disease. Clin. Chem. Lab Med. 51, 317–325 (2013).
Borgono, C. A. & Diamandis, E. P. The emerging roles of human tissue kallikreins in cancer. Nat. Rev. Cancer 4, 876–890 (2004).
Sotiropoulou, G., Pampalakis, G. & Diamandis, E. P. Functional roles of human kallikrein-related peptidases. J. Biol. Chem. 284, 32989–32994 (2009).
Oikonomopoulou, K., Diamandis, E. P. & Hollenberg, M. D. Kallikrein-related peptidases: proteolysis and signaling in cancer, the new frontier. Biol. Chem. 391, 299–310 (2010).
Moore, A. R. et al. Destruction of articular cartilage by alpha2 macroglobulin elastase complexes: role in rheumatoid arthritis. Ann. Rheum. Dis. 58, 109–113 (1999).
Miyata, J. et al. Cathepsin G: the significance in rheumatoid arthritis as a monocyte chemoattractant. Rheumatol. Int. 27, 375–382 (2007).
Milner, J. M. et al. Matriptase is a novel initiator of cartilage matrix degradation in osteoarthritis. Arthritis Rheum. 62, 1955–1966 (2010).
Wilkinson, D. J. et al. Matriptase induction of metalloproteinase-dependent aggrecanolysis in vitro and in vivo: promotion of osteoarthritic cartilage damage by multiple mechanisms. Arthritis Rheumatol. 69, 1601–1611 (2017).
Nigrovic, P. A. & Lee, D. M. Synovial mast cells: role in acute and chronic arthritis. Immunol. Rev. 217, 19–37 (2007).
Nakano, S. et al. Distinct expression of mast cell tryptase and protease activated receptor-2 in synovia of rheumatoid arthritis and osteoarthritis. Clin. Rheumatol. 26, 1284–1292 (2007).
Buckley, M. G. et al. Mast cell activation in arthritis: detection of α- and β-tryptase, histamine and eosinophil cationic protein in synovial fluid. Clin. Sci. (Lond.) 93, 363–370 (1997).
Ricard-Blum, S. & Vallet, S. D. Proteases decode the extracellular matrix cryptome. Biochimie 122, 300–313 (2016).
Kon, S. et al. A novel cryptic binding motif, LRSKSRSFQVSDEQY, in the C-terminal fragment of MMP-3/7-cleaved osteopontin as a novel ligand for α9β1 integrin is involved in the anti-type II collagen antibody-induced arthritis. PLoS ONE 9, e116210 (2014).
Williams, R. J. 3rd, Smith, R. L. & Schurman, D. J. Septic arthritis. Staphylococcal induction of chondrocyte proteolytic activity. Arthritis Rheum. 33, 533–541 (1990).
Konig, M. F. et al. Defining the role of Porphyromonas gingivalis peptidylarginine deiminase (PPAD) in rheumatoid arthritis through the study of PPAD biology. Ann. Rheum. Dis. 74, 2054–2061 (2015).
Maresz, K. J. et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 9, e1003627 (2013).
Holzhausen, M., Spolidorio, L. C. & Vergnolle, N. Role of protease-activated receptor-2 in inflammation, and its possible implications as a putative mediator of periodontitis. Mem. Inst. Oswaldo Cruz 100 (Suppl. 1), 177–180 (2005).
Ramachandran, R., Altier, C., Oikonomopoulou, K. & Hollenberg, M. D. Proteinases, their extracellular targets, and inflammatory signaling. Pharmacol. Rev. 68, 1110–1142 (2016).
Neumann, E., Khawaja, K. & Muller-Ladner, U. G protein-coupled receptors in rheumatology. Nat. Rev. Rheumatol. 10, 429–436 (2014).
Adams, M. N. et al. Structure, function and pathophysiology of protease activated receptors. Pharmacol. Ther. 130, 248–282 (2011).
Morris, R., Winyard, P. G., Brass, L. F., Blake, D. R. & Morris, C. J. Thrombin receptor expression in rheumatoid and osteoarthritic synovial tissue. Ann. Rheum. Dis. 55, 841–843 (1996).
Xiang, Y. et al. Expression of proteinase-activated receptors (PAR)-2 in articular chondrocytes is modulated by IL-1β, TNF-α and TGF-βa. Osteoarthritis Cartilage 14, 1163–1173 (2006).
Busso, N. et al. Evaluation of protease-activated receptor 2 in murine models of arthritis. Arthritis Rheum. 56, 101–107 (2007).
Hirano, F. et al. Thrombin-induced expression of RANTES mRNA through protease activated receptor-1 in human synovial fibroblasts. Ann. Rheum. Dis. 61, 834–837 (2002).
Boileau, C. et al. Activation of proteinase-activated receptor 2 in human osteoarthritic cartilage upregulates catabolic and proinflammatory pathways capable of inducing cartilage degradation: a basic science study. Arthritis Res. Ther. 9, R121 (2007).
Abraham, L. A. et al. Expression of protease-activated receptor-2 by osteoblasts. Bone 26, 7–14 (2000).
Lam, F. F. Role of protease-activated receptor 2 in joint inflammation. Arthritis Rheum. 56, 3514–3517 (2007).
Palmer, H. S. et al. Protease-activated receptor 2 mediates the proinflammatory effects of synovial mast cells. Arthritis Rheum. 56, 3532–3540 (2007).
Jackson, M. T. et al. Depletion of protease-activated receptor 2 but not protease-activated receptor 1 may confer protection against osteoarthritis in mice through extracartilaginous mechanisms. Arthritis Rheumatol. 66, 3337–3348 (2014).
Crilly, A. et al. Immunomodulatory role of proteinase-activated receptor-2. Ann. Rheum. Dis. 71, 1559–1566 (2012).
McDougall, J. J. et al. Triggering of proteinase-activated receptor 4 leads to joint pain and inflammation in mice. Arthritis Rheum. 60, 728–737 (2009).
Ferrell, W. R. et al. Essential role for proteinase-activated receptor-2 in arthritis. J. Clin. Invest. 111, 35–41 (2003).
Kelso, E. B. et al. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J. Pharmacol. Exp. Ther. 316, 1017–1024 (2006).
Xue, M. et al. Protease-activated receptor 2, rather than protease-activated receptor 1, contributes to the aggressive properties of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 64, 88–98 (2012).
Tindell, A. G. et al. Correlation of protease-activated receptor-2 expression and synovitis in rheumatoid and osteoarthritis. Rheumatol. Int. 32, 3077–3086 (2012).
Amiable, N. et al. Proteinase-activated receptor-2 gene disruption limits the effect of osteoarthritis on cartilage in mice: a novel target in joint degradation. J. Rheumatol. 38, 911–920 (2011).
Ferrell, W. R., Kelso, E. B., Lockhart, J. C., Plevin, R. & McInnes, I. B. Protease-activated receptor 2: a novel pathogenic pathway in a murine model of osteoarthritis. Ann. Rheum. Dis. 69, 2051–2054 (2010).
Yang, Y. H. et al. Reduction of arthritis severity in protease-activated receptor-deficient mice. Arthritis Rheum. 52, 1325–1332 (2005).
Kelso, E. B. et al. Expression and proinflammatory role of proteinase-activated receptor 2 in rheumatoid synovium: ex vivo studies using a novel proteinase-activated receptor 2 antagonist. Arthritis Rheum. 56, 765–771 (2007).
McInnes, I. B. & Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7, 429–442 (2007).
Wojdasiewicz, P., Poniatowski, L. A. & Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014, 561459 (2014).
McDougall, J. J. Involvement of proteinase-activated receptor-4 in inflammatory joint disease. Inflamm. Res. 56, S354 (2007).
Busso, N. et al. Essential role of platelet activation via protease activated receptor 4 in tissue factor-initiated inflammation. Arthritis Res. Ther. 10, R42 (2008).
Weinberg, J. B., Pippen, A. M. & Greenberg, C. S. Extravascular fibrin formation and dissolution in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 34, 996–1005 (1991).
Klos, A., Wende, E., Wareham, K. J. & Monk, P. N. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXVII. Complement peptide C5a, C4a, and C3a receptors. Pharmacol. Rev. 65, 500–543 (2013).
Grant, E. P. et al. Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis. J. Exp. Med. 196, 1461–1471 (2002).
Oikonomopoulou, K. et al. Proteinase-activated receptors, targets for kallikrein signaling. J. Biol. Chem. 281, 32095–32112 (2006).
Vergnolle, N. Protease-activated receptors as drug targets in inflammation and pain. Pharmacol. Ther. 123, 292–309 (2009).
McDougall, J. J. Arthritis and pain. Neurogenic origin of joint pain. Arthritis Res. Ther. 8, 220 (2006).
McDougall, J. J. & Linton, P. Neurophysiology of arthritis pain. Curr. Pain Headache Rep. 16, 485–491 (2012).
Huesa, C. et al. Proteinase-activated receptor 2 modulates OA-related pain, cartilage and bone pathology. Ann. Rheum. Dis. 75, 1989–1997 (2016).
Muley, M. M. et al. Neutrophil elastase induces inflammation and pain in mouse knee joints via activation of proteinase-activated receptor-2. Br. J. Pharmacol. 173, 766–777 (2016).
Russell, F. A. & McDougall, J. J. Proteinase activated receptor (PAR) involvement in mediating arthritis pain and inflammation. Inflamm. Res. 58, 119–126 (2009).
Martin, L. et al. Thrombin receptor: an endogenous inhibitor of inflammatory pain, activating opioid pathways. Pain 146, 121–129 (2009).
Asfaha, S., Brussee, V., Chapman, K., Zochodne, D. W. & Vergnolle, N. Proteinase-activated receptor-1 agonists attenuate nociception in response to noxious stimuli. Br. J. Pharmacol. 135, 1101–1106 (2002).
Kawabata, A., Kawao, N., Kuroda, R., Tanaka, A. & Shimada, C. The PAR-1-activating peptide attenuates carrageenan-induced hyperalgesia in rats. Peptides 23, 1181–1183 (2002).
Steinhoff, M. et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 6, 151–158 (2000).
Helyes, Z. et al. Involvement of transient receptor potential vanilloid 1 receptors in protease-activated receptor-2-induced joint inflammation and nociception. Eur. J. Pain 14, 351–358 (2010).
Russell, F. A., Schuelert, N., Veldhoen, V. E., Hollenberg, M. D. & McDougall, J. J. Activation of PAR(2) receptors sensitizes primary afferents and causes leukocyte rolling and adherence in the rat knee joint. Br. J. Pharmacol. 167, 1665–1678 (2012).
Russell, F. A., Veldhoen, V. E., Tchitchkan, D. & McDougall, J. J. Proteinase-activated receptor-4 (PAR4) activation leads to sensitization of rat joint primary afferents via a bradykinin B2 receptor-dependent mechanism. J. Neurophysiol. 103, 155–163 (2010).
Russell, F. A. et al. The pronociceptive effect of proteinase-activated receptor-4 stimulation in rat knee joints is dependent on mast cell activation. Pain 152, 354–360 (2011).
Chen, Y., Yang, C. & Wang, Z. J. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience 193, 440–451 (2011).
Poole, D. P. et al. Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J. Biol. Chem. 288, 5790–5802 (2013).
Vellani, V. et al. Protease activated receptors 1 and 4 sensitize TRPV1 in nociceptive neurones. Mol. Pain 6, 61 (2010).
O'Conor, C. J., Leddy, H. A., Benefield, H. C., Liedtke, W. B. & Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl Acad. Sci. USA 111, 1316–1321 (2014).
Guilak, F., Leddy, H. A. & Liedtke, W. Transient receptor potential vanilloid 4: The sixth sense of the musculoskeletal system? Ann. NY Acad. Sci. 1192, 404–409 (2010).
Lamande, S. R. et al. Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nat. Genet. 43, 1142–1146 (2011).
Grant, A. D. et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. 578, 715–733 (2007).
Saifeddine, M. et al. GPCR-mediated EGF receptor transactivation regulates TRPV4 action in the vasculature. Br. J. Pharmacol. 172, 2493–2506 (2015).
Schaible, H. G. Nociceptive neurons detect cytokines in arthritis. Arthritis Res. Ther. 16, 470 (2014).
Ramachandran, R. et al. Neutrophil elastase acts as a biased agonist for proteinase-activated receptor-2 (PAR2). J. Biol. Chem. 286, 24638–24648 (2011).
Mihara, K., Ramachandran, R., Renaux, B., Saifeddine, M. & Hollenberg, M. D. Neutrophil elastase and proteinase-3 trigger G protein-biased signaling through proteinase-activated receptor-1 (PAR1). J. Biol. Chem. 288, 32979–32990 (2013).
Hollenberg, M. D. et al. Biased signalling and proteinase-activated receptors (PARs): targeting inflammatory disease. Br. J. Pharmacol. 171, 1180–1194 (2014).
Zhao, P., Metcalf, M. & Bunnett, N. W. Biased signaling of protease-activated receptors. Front. Endocrinol. (Lausanne) 5, 67 (2014).
Zhao, P. et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J. Biol. Chem. 289, 27215–27234 (2014).
Sostegni, S. et al. Sensitisation of TRPV4 by PAR2 is independent of intracellular calcium signalling and can be mediated by the biased agonist neutrophil elastase. Pflugers Arch. 467, 687–701 (2015).
Haerteis, S. et al. Proteolytic activation of the epithelial sodium channel (ENaC) by the cysteine protease cathepsin-S. Pflugers Arch. 464, 353–365 (2012).
Mobasheri, A., Barrett-Jolley, R., Shakibaei, M., Canessa, C. M. & Martin-Vasallo, P. in Mechanosensitivity in Cells and Tissues Ch. 20 (eds Kamkin, A. & Kiseleva, I.) (Academia, 2005).
Clark, A. K. & Malcangio, M. Microglial signalling mechanisms: cathepsin S and fractalkine. Exp. Neurol. 234, 283–292 (2012).
Stefansson, K. et al. Activation of proteinase-activated receptor-2 by human kallikrein-related peptidases. J. Invest. Dermatol. 128, 18–25 (2008).
Ramachandran, R., Noorbakhsh, F., DeFea, K. & Hollenberg, M. D. Targeting proteinase-activated receptors: therapeutic potential and challenges. Nat. Rev. Drug Discov. 11, 69–86 (2012).
Ramsay, A. J. et al. Kallikrein-related peptidase 4 (KLK4) initiates intracellular signaling via protease-activated receptors (PARs). KLK4 and PAR-2 are co-expressed during prostate cancer progression. J. Biol. Chem. 283, 12293–12304 (2008).
Gratio, V. et al. Kallikrein-related peptidase 4: a new activator of the aberrantly expressed protease-activated receptor 1 in colon cancer cells. Am. J. Pathol. 176, 1452–1461 (2010).
Briot, A. et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J. Exp. Med. 206, 1135–1147 (2009).
Boire, A. et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120, 303–313 (2005).
Blackburn, J. S. & Brinckerhoff, C. E. Matrix metalloproteinase-1 and thrombin differentially activate gene expression in endothelial cells via PAR-1 and promote angiogenesis. Am. J. Pathol. 173, 1736–1746 (2008).
Trivedi, V. et al. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell 137, 332–343 (2009).
Tressel, S. L. et al. A matrix metalloprotease-PAR1 system regulates vascular integrity, systemic inflammation and death in sepsis. EMBO Mol. Med. 3, 370–384 (2011).
Holzhausen, M. et al. Protease-activated receptor-2 activation: a major role in the pathogenesis of Porphyromonas gingivalis infection. Am. J. Pathol. 168, 1189–1199 (2006).
Lourbakos, A. et al. Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: a new trait in microbial pathogenicity. Blood 97, 3790–3797 (2001).
Dommisch, H. et al. Protease-activated receptor 2 mediates human β-defensin 2 and CC chemokine ligand 20 mRNA expression in response to proteases secreted by Porphyromonas gingivalis. Infect. Immun. 75, 4326–4333 (2007).
Euzebio Alves, V. T. et al. Periodontal treatment downregulates protease-activated receptor 2 in human gingival crevicular fluid cells. Infect. Immun. 81, 4399–4407 (2013).
Chung, W. O., Hansen, S. R., Rao, D. & Dale, B. A. Protease-activated receptor signaling increases epithelial antimicrobial peptide expression. J. Immunol. 173, 5165–5170 (2004).
Totaro, M. C. et al. Porphyromonas gingivalis and the pathogenesis of rheumatoid arthritis: analysis of various compartments including the synovial tissue. Arthritis Res. Ther. 15, R66 (2013).
Potempa, J., Mydel, P. & Koziel, J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 13, 606–620 (2017).
Kuruvilla, M. & Gurk-Turner, C. A review of warfarin dosing and monitoring. Proc. (Bayl. Univ. Med. Cent.) 14, 305–306 (2001).
Chen, B. et al. Characterization of thrombin-bound dabigatran effects on protease-activated receptor-1 expression and signaling in vitro. Mol. Pharmacol. 88, 95–105 (2015).
Morgan, B. P. & Harris, C. L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 14, 857–877 (2015).
Turk, B. Targeting proteases: successes, failures and future prospects. Nat. Rev. Drug Discov. 5, 785–799 (2006).
Fingleton, B. MMPs as therapeutic targets—still a viable option? Semin. Cell Dev. Biol. 19, 61–68 (2008).
Liu, J. & Khalil, R. A. Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog. Mol. Biol. Transl. Sci. 148, 355–420 (2017).
Close, D. R. Matrix metalloproteinase inhibitors in rheumatic diseases. Ann. Rheum. Dis. 60 (Suppl 3), iii62–ii67 (2001).
Erin, E. M. et al. Effects of a reversible β-tryptase and trypsin inhibitor (RWJ-58643) on nasal allergic responses. Clin. Exp. Allergy 36, 458–464 (2006).
Hemmings, F. J., Farhan, M., Rowland, J., Banken, L. & Jain, R. Tolerability and pharmacokinetics of the collagenase-selective inhibitor Trocade in patients with rheumatoid arthritis. Rheumatology (Oxford) 40, 537–543 (2001).
Brandt, K. D. et al. Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 52, 2015–2025 (2005).
Jensen, M. R. et al. Structural basis for simvastatin competitive antagonism of complement receptor 3. J. Biol. Chem. 291, 16963–16976 (2016).
Goto, S. & Tomita, A. New antithrombotics for secondary prevention of acute coronary syndrome. Clin. Cardiol. 37, 178–187 (2014).
Pan H., Boucher, M. & Kaunelis, D. in CADTH Issues in Emerging Health Technologies Ch.148 (Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, 2016).
Yau, M. K., Liu, L. & Fairlie, D. P. Toward drugs for protease-activated receptor 2 (PAR2). J. Med. Chem. 56, 7477–7497 (2013).
Kuliopulos, A. & Covic, L. Blocking receptors on the inside: pepducin-based intervention of PAR signaling and thrombosis. Life Sci. 74, 255–262 (2003).
Gurbel, P. A. et al. Cell-penetrating pepducin therapy targeting PAR1 in subjects with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 36, 189–197 (2016).
Sevigny, L. M. et al. Interdicting protease-activated receptor-2-driven inflammation with cell-penetrating pepducins. Proc. Natl Acad. Sci. USA 108, 8491–8496 (2011).
Alessandri-Haber, N., Dina, O. A., Joseph, E. K., Reichling, D. & Levine, J. D. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J. Neurosci. 26, 3864–3874 (2006).
Patapoutian A., Tate, S. & Woolf, C. J. Transient receptor potential channels: targeting pain at the source. Nat. Rev. Drug Discov. 8, 55–68 (2009).
Kanju, P. et al. Small molecule dual-inhibitors of TRPV4 and TRPA1 for attenuation of inflammation and pain. Sci. Rep. 6, 26894 (2016).
Acknowledgements
The authors thank Sowmya Viswanathan, Konstantinos Tselios and Alejandro Gómez-Aristizábal at the University Health Network, Toronto, Canada for critical discussions of this manuscript. The authors' ongoing work related to this field of research is supported by the Krembil Foundation (K.O. and V.C.) and the Canadian Institutes of Health Research (E.P.D. and M.D.H.).
Author information
Authors and Affiliations
Contributions
K.O. and V.C. researched the data for this article. All authors wrote this article, made substantial contributions to discussions of content and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Oikonomopoulou, K., Diamandis, E., Hollenberg, M. et al. Proteinases and their receptors in inflammatory arthritis: an overview. Nat Rev Rheumatol 14, 170–180 (2018). https://doi.org/10.1038/nrrheum.2018.17
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2018.17
This article is cited by
-
Prokineticin 2 is a catabolic regulator of osteoarthritic cartilage destruction in mouse
Arthritis Research & Therapy (2023)
-
Disease-microenvironment modulation by bare- or engineered-exosome for rheumatoid arthritis treatment
Biomaterials Research (2023)
-
Human osteoarthritis knee joint synovial fluids cleave and activate Proteinase-Activated Receptor (PAR) mediated signaling
Scientific Reports (2023)
-
Kartogenin prevents cartilage degradation and alleviates osteoarthritis progression in mice via the miR-146a/NRF2 axis
Cell Death & Disease (2021)
-
A20: a master regulator of arthritis
Arthritis Research & Therapy (2020)