Key Points
-
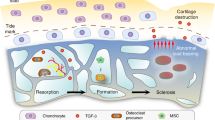
Osteoarthritis (OA) is a whole-joint disease in which all of the components of the joint are affected
-
The articular cartilage, subchondral bone, and calcified cartilage form an osteochondral biocomposite that is uniquely adapted to transferring loads during weight bearing and joint motion
-
Marked alterations in the composition, functional properties, and structure of the osteochondral tissues occur during the evolution of OA
-
Alteration in the composition or structure of any of the individual components of the osteochondral unit can initiate OA pathology in the joint
-
The differential capacity of bone and cartilage to adapt to the effects of local mechanical and environmental influences has an important role in the development of OA
-
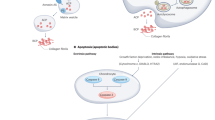
Crosstalk between chondrocytes and bone cells contributes to OA pathogenesis
-
To develop rational therapies for OA, it is essential to have diagnostic tools to define the state of the components of the osteochondral unit
Abstract
In diarthrodial joints, the articular cartilage, calcified cartilage, and subchondral cortical and trabecular bone form a biocomposite — referred to as the osteochondral unit — that is uniquely adapted to the transfer of load. During the evolution of the osteoarthritic process the compositions, functional properties, and structures of these tissues undergo marked alterations. Although pathological processes might selectively target a single joint tissue, ultimately all of the components of the osteochondral unit will be affected because of their intimate association, and thus the biological and physical crosstalk among them is of great importance. The development of targeted therapies against the osteoarthritic processes in cartilage or bone will, therefore, require an understanding of the state of these joint tissues at the time of the intervention. Importantly, these interventions will not be successful unless they are applied at the early stages of disease before considerable structural and functional alterations occur in the osteochondral unit. This Review describes the changes that occur in bone and cartilage during the osteoarthritic process, and highlights strategies for how this knowledge could be applied to develop new therapeutic interventions for osteoarthritis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Goldring, S. R. & Goldring, M. B. in Kelley's Textbook of Rheumatology 9th edn Ch. 1 (eds Firestein, G.S., Budd, R.C., Gabriel, S.E., McInnes I.B., & O'Dell J.R.) 1–19 (Saunders, 2013).
Johnson, V. L. & Hunter, D. J. The epidemiology of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 28, 5–15 (2014).
Hunter, D. J., Nevitt, M., Losina, E. & Kraus, V. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract. Res. Clin. Rheumatol. 28, 61–71 (2014).
Loeser, R. F., Goldring, S. R., Scanzello, C. R. & Goldring, M. B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64, 1697–1707 (2012).
Heinegard, D. & Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 7, 50–56 (2011).
Hunziker, E. B., Lippuner, K. & Shintani, N. How best to preserve and reveal the structural intricacies of cartilaginous tissue. Matrix Biol. 39, 33–43 (2014).
Onnerfjord, P., Khabut, A., Reinholt, F. P., Svensson, O. & Heinegard, D. Quantitative proteomic analysis of eight cartilaginous tissues reveals characteristic differences as well as similarities between subgroups. J. Biol. Chem. 287, 18913–18924 (2012).
Houard, X., Goldring, M. B. & Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 15, 375 (2013).
Quinn, T. M., Hauselmann, H. J., Shintani, N. & Hunziker, E. B. Cell and matrix morphology in articular cartilage from adult human knee and ankle joints suggests depth-associated adaptations to biomechanical and anatomical roles. Osteoarthritis Cartilage 21, 1904–1912 (2013).
Sandell, L. J. Etiology of osteoarthritis: genetics and synovial joint development. Nat. Rev. Rheumatol. 8, 77–89 (2012).
Andriacchi, T. P. & Favre, J. The nature of in vivo mechanical signals that influence cartilage health and progression to knee osteoarthritis. Curr. Rheumatol. Rep. 16, 463 (2014).
Guo, H., Maher, S. A. & Torzilli, P. A. A biphasic finite element study on the role of the articular cartilage superficial zone in confined compression. J. Biomech. 48, 166–170 (2015).
Greene, G. W. et al. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proc. Natl. Acad. Sci. USA 108, 5255–5259 (2011).
Imhof, H. et al. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest. Radiol. 35, 581–588 (2000).
Lyons, T. J., McClure, S. F., Stoddart, R. W. & McClure, J. The normal human chondro-osseous junctional region: evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Musculoskelet. Disord. 7, 52 (2006).
Pan, J. et al. In situ measurement of transport between subchondral bone and articular cartilage. J. Orthop. Res. 27, 1347–1352 (2009).
Hunziker, E. B., Kapfinger, E. & Geiss, J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage 15, 403–413 (2007).
Wilusz, R. E., Sanchez-Adams, J. & Guilak, F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 39, 25–32 (2014).
Pap, T. & Bertrand, J. Syndecans in cartilage breakdown and synovial inflammation. Nat. Rev. Rheumatol. 9, 43–55 (2013).
Loeser, R. F. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol. 39, 11–16 (2014).
Xu, L., Golshirazian, I., Asbury, B. J. & Li, Y. Induction of high temperature requirement A1, a serine protease, by TGF-β1 in articular chondrocytes of mouse models of OA. Histol. Histopathol. 29, 609–618 (2014).
Wann, A. K. et al. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. 26, 1663–1671 (2012).
Ruhlen, R. & Marberry, K. The chondrocyte primary cilium. Osteoarthritis Cartilage 22, 1071–1076 (2014).
Mobasheri, A. et al. Facilitative glucose transporters in articular chondrocytes. Expression, distribution and functional regulation of GLUT isoforms by hypoxia, hypoxia mimetics, growth factors and pro-inflammatory cytokines. Adv. Anat. Embryol. Cell Biol. 200, 1–84 (2008).
Pfander, D. & Gelse, K. Hypoxia and osteoarthritis: how chondrocytes survive hypoxic environments. Curr. Opin. Rheumatol. 19, 457–462 (2007).
Thoms, B. L., Dudek, K. A., Lafont, J. E. & Murphy, C. L. Hypoxia promotes the production and inhibits the destruction of human articular cartilage. Arthritis Rheum. 65, 1302–1312 (2013).
Maes, C. et al. VEGF-independent cell-autonomous functions of HIF-1α regulating oxygen consumption in fetal cartilage are critical for chondrocyte survival. J. Bone Miner. Res. 27, 596–609 (2012).
Simkin, P. A. Consider the tidemark. J. Rheumatol. 39, 890–892 (2012).
Burr, D. B. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage 12 (Suppl A), S20–S30 (2004).
Burr, D. B. & Gallant, M. A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 8, 665–673 (2012).
Goldring, S. R. Role of bone in osteoarthritis pathogenesis. Med. Clin. North Am. 93, 25–35 (2009).
Goldring, M. B. & Goldring, S. R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 1192, 230–237 (2010).
Benjamin, M. & McGonagle, D. The enthesis organ concept and its relevance to the spondyloarthropathies. Adv. Exp. Med. Biol. 649, 57–70 (2009).
Frost, H. M. From Wolff's law to the Utah paradigm: insights about bone physiology and its clinical applications. Anat. Rec. 262, 398–419 (2001).
Eriksen, E. F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 11, 219–227 (2010).
Xiong, J. et al. Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241 (2011).
Nakashima, T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 (2011).
Dallas, S. L., Prideaux, M. & Bonewald, L. F. The osteocyte: an endocrine cell... and more. Endocr. Rev. 34, 658–690 (2013).
Meunier, P. J. & Boivin, G. Bone mineral density reflects bone mass but also the degree of mineralization of bone: therapeutic implications. Bone 21, 373–377 (1997).
Faibish, D., Ott, S. M. & Boskey, A. L. Mineral changes in osteoporosis: a review. Clin. Orthop. Relat. Res. 443, 28–38 (2006).
Day, J. S. et al. Adaptation of subchondral bone in osteoarthritis. Biorheology 41, 359–368 (2004).
Day, J. S. et al. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J. Orthop. Res. 19, 914–918 (2001).
Goldring, M. B. & Goldring, S. R. Osteoarthritis. J. Cell. Physiol. 213, 626–634 (2007).
Akizuki, S., Mow, V. C., Muller, F., Pita, J. C. & Howell, D. S. Tensile properties of human knee joint cartilage. II. Correlations between weight bearing and tissue pathology and the kinetics of swelling. J. Orthop. Res. 5, 173–186 (1987).
Roberts, S., Weightman, B., Urban, J. & Chappell, D. Mechanical and biochemical properties of human articular cartilage in osteoarthritic femoral heads and in autopsy specimens. J. Bone Joint Surg. Br. 68, 278–288 (1986).
Calvo, E. et al. Histopathological correlation of cartilage swelling detected by magnetic resonance imaging in early experimental osteoarthritis. Osteoarthritis Cartilage 12, 878–886 (2004).
Wu, W. et al. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 46, 2087–2094 (2002).
Fosang, A. J. & Beier, F. Emerging frontiers in cartilage and chondrocyte biology. Best Pract. Res. Clin. Rheumatol. 25, 751–766 (2011).
Eyre, D. R. Collagens and cartilage matrix homeostasis. Clin. Orthop. Relat. Res. 427 (Suppl.), S118–S122 (2004).
Wang, M. et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res. Ther. 15, R5 (2013).
Scanzello, C. R. & Goldring, S. R. The role of synovitis in osteoarthritis pathogenesis. Bone 51, 249–257 (2012).
Loeser, R. F. Aging processes and the development of osteoarthritis. Curr. Opin. Rheumatol. 25, 108–113 (2013).
Pritzker, K. P. et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage 14, 13–29 (2006).
Zhou, S. et al. Influence of osteoarthritis grade on molecular signature of human cartilage. J. Orthop. Res. 34, 454–462 (2016).
Radin, E. L. et al. Effects of mechanical loading on the tissues of the rabbit knee. J. Orthop. Res. 2, 221–234 (1984).
Lorenzo, P., Bayliss, M. T. & Heinegard, D. Altered patterns and synthesis of extracellular matrix macromolecules in early osteoarthritis. Matrix Biol. 23, 381–391 (2004).
Rolauffs, B. et al. Proliferative remodeling of the spatial organization of human superficial chondrocytes distant from focal early osteoarthritis. Arthritis Rheum. 62, 489–498 (2010).
McGlashan, S. R., Cluett, E. C., Jensen, C. G. & Poole, C. A. Primary cilia in osteoarthritic chondrocytes: from chondrons to clusters. Dev. Dyn. 237, 2013–2020 (2008).
Hwang, H. S. & Kim, H. A. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 16, 26035–26054 (2015).
Liu-Bryan, R. & Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 11, 35–44 (2015).
Walsh, D. A. et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 49, 1852–1861 (2010).
Suri, S. & Walsh, D. A. Osteochondral alterations in osteoarthritis. Bone 51, 204–211 (2012).
Saito, T. et al. Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat. Med. 16, 678–686 (2010).
Yang, S. et al. Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 16, 687–693 (2010).
Hashimoto, K. et al. Regulated transcription of human matrix metalloproteinase 13 (MMP13) and interleukin-1β (IL1B) genes in chondrocytes depends on methylation of specific proximal promoter CpG sites. J. Biol. Chem. 288, 10061–10072 (2013).
Ruan, M. Z. et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci. Transl. Med. 5, 176ra134 (2013).
Kim, J. H. et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell 156, 730–743 (2014).
Lane, L. B., Villacin, A. & Bullough, P. G. The vascularity and remodelling of subchondrial bone and calcified cartilage in adult human femoral and humeral heads. An age- and stress-related phenomenon. J. Bone Joint Surg. Br. 59, 272–278 (1977).
Burr, D. B. & Schaffler, M. B. The involvement of subchondral mineralized tissues in osteoarthrosis: quantitative microscopic evidence. Microsc. Res. Tech. 37, 343–357 (1997).
Bullough, P. G. The role of joint architecture in the etiology of arthritis. Osteoarthritis Cartilage 12 (Suppl. A), S2–S9 (2004).
Walsh, D. A. et al. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage 15, 743–751 (2007).
Walsh, D. A. Angiogenesis in osteoarthritis and spondylosis: successful repair with undesirable outcomes. Curr. Opin. Rheumatol. 16, 609–615 (2004).
Suri, S. et al. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann. Rheum. Dis. 66, 1423–1428 (2007).
Ashraf, S. et al. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann. Rheum. Dis. 70, 523–529 (2011).
Ashraf, S. & Walsh, D. A. Angiogenesis in osteoarthritis. Curr. Opin. Rheumatol. 20, 573–580 (2008).
Reichenbach, S. et al. Prevalence of bone attrition on knee radiographs and MRI in a community-based cohort. Osteoarthritis Cartilage 16, 1005–1010 (2008).
Neogi, T. et al. Subchondral bone attrition may be a reflection of compartment-specific mechanical load: the MOST Study. Ann. Rheum. Dis. 69, 841–844 (2010).
Goldring, S. R. The role of bone in osteoarthritis pathogenesis. Rheum. Dis. Clin. North Am. 34, 561–571 (2008).
Buckland-Wright, C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthritis Cartilage 12 (Suppl. A), S10–S19 (2004).
Buckland-Wright, J. C., Messent, E. A., Bingham, C. O. 3rd, Ward, R. J. & Tonkin, C. A 2 yr longitudinal radiographic study examining the effect of a bisphosphonate (risedronate) upon subchondral bone loss in osteoarthritic knee patients. Rheumatology (Oxford) 46, 257–264 (2007).
Dieppe, P., Cushnaghan, J., Young, P. & Kirwan, J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann. Rheum. Dis. 52, 557–563 (1993).
Hutton, C. W., Higgs, E. R., Jackson, P. C., Watt, I. & Dieppe, P. A. 99mTc HMDP bone scanning in generalised nodal osteoarthritis. II. The four hour bone scan image predicts radiographic change. Ann. Rheum. Dis. 45, 622–626 (1986).
Kim, H. R., So, Y., Moon, S. G., Lee, I. S. & Lee, S. H. Clinical value of 99mTc-methylene diphosphonate (MDP) bone single photon emission computed tomography (SPECT) in patients with knee osteoarthritis. Osteoarthritis Cartilage 16, 212–218 (2008).
Addison, S., Coleman, R. E., Feng, S., McDaniel, G. & Kraus, V. B. Whole-body bone scintigraphy provides a measure of the total-body burden of osteoarthritis for the purpose of systemic biomarker validation. Arthritis Rheum. 60, 3366–3373 (2009).
Anderson, D. D. et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J. Orthop. Res. 29, 802–809 (2011).
Kuroki, K., Cook, C. R. & Cook, J. L. Subchondral bone changes in three different canine models of osteoarthritis. Osteoarthritis Cartilage 19, 1142–1149 (2011).
Fahlgren, A., Messner, K. & Aspenberg, P. Meniscectomy leads to an early increase in subchondral bone plate thickness in the rabbit knee. Acta Orthop. Scand. 74, 437–441 (2003).
Botter, S. M. et al. Cartilage damage pattern in relation to subchondral plate thickness in a collagenase-induced model of osteoarthritis. Osteoarthritis Cartilage 16, 506–514 (2008).
Pauly, H. M. et al. Assessment of cortical and trabecular bone changes in two models of post-traumatic osteoarthritis. J. Orthop. Res. 33, 1835–1845 (2015).
Ko, F. C. et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 65, 1569–1578 (2013).
Poulet, B., Hamilton, R. W., Shefelbine, S. & Pitsillides, A. A. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 63, 137–147 (2011).
Brown, T. D., Radin, E. L., Martin, R. B. & Burr, D. B. Finite element studies of some juxtarticular stress changes due to localized subchondral stiffening. J. Biomech. 17, 11–24 (1984).
Karvonen, R. L., Miller, P. R., Nelson, D. A., Granda, J. L. & Fernandez-Madrid, F. Periarticular osteoporosis in osteoarthritis of the knee. J. Rheumatol. 25, 2187–2194 (1998).
Wilson, A. J., Murphy, W. A., Hardy, D. C. & Totty, W. G. Transient osteoporosis: transient bone marrow edema? Radiology 167, 757–760 (1988).
Taljanovic, M. S. et al. Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal Radiol. 37, 423–431 (2008).
Leydet-Quilici, H. et al. Advanced hip osteoarthritis: magnetic resonance imaging aspects and histopathology correlations. Osteoarthritis Cartilage 18, 1429–1435 (2010).
Zanetti, M., Bruder, E., Romero, J. & Hodler, J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology 215, 835–840 (2000).
Kothari, A. et al. Within-subregion relationship between bone marrow lesions and subsequent cartilage loss in knee osteoarthritis. Arthritis Care Res. (Hoboken) 62, 198–203 (2010).
Roemer, F. W., Hunter, D. J. & Guermazi, A. MRI-based semiquantitative assessment of subchondral bone marrow lesions in osteoarthritis research. Osteoarthritis Cartilage 17, 414–415 (2009).
Roemer, F. W. et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann. Rheum. Dis. 70, 1804–1809 (2011).
Roemer, F. W. et al. What comes first? Multitissue involvement leading to radiographic osteoarthritis: magnetic resonance imaging-based trajectory analysis over four years in the osteoarthritis initiative. Arthritis Rheumatol. 67, 2085–2096 (2015).
Hunter, D. J. et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 54, 1529–1535 (2006).
Felson, D. T. et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann. Intern. Med. 139, 330–336 (2003).
Hernandez-Molina, G. et al. The association of bone attrition with knee pain and other MRI features of osteoarthritis. Ann. Rheum. Dis. 67, 43–47 (2008).
Lo, G. H., Hunter, D. J., Nevitt, M., Lynch, J. & McAlindon, T. E. Strong association of MRI meniscal derangement and bone marrow lesions in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage 17, 743–747 (2009).
Bowes, M. A. et al. Osteoarthritic bone marrow lesions almost exclusively colocate with denuded cartilage: a 3D study using data from the Osteoarthritis Initiative. Ann. Rheum. Dis., http://dx.doi.org/10.1136/annrheumdis-2015-208407 (2015).
Sokoloff, L. Microcracks in the calcified layer of articular cartilage. Arch. Pathol. Lab. Med. 117, 191–195 (1993).
Lacourt, M. et al. Relationship between cartilage and subchondral bone lesions in repetitive impact trauma-induced equine osteoarthritis. Osteoarthritis Cartilage 20, 572–583 (2012).
Norrdin, R. W. & Stover, S. M. Subchondral bone failure in overload arthrosis: a scanning electron microscopic study in horses. J. Musculoskelet. Neuronal Interact. 6, 251–257 (2006).
Boyde, A. & Firth, E. C. High resolution microscopic survey of third metacarpal articular calcified cartilage and subchondral bone in the juvenile horse: possible implications in chondro-osseous disease. Microsc. Res. Tech. 71, 477–488 (2008).
Mori, S. & Burr, D. B. Increased intracortical remodeling following fatigue damage. Bone 14, 103–109 (1993).
Mori, S., Harruff, R. & Burr, D. B. Microcracks in articular calcified cartilage of human femoral heads. Arch. Pathol. Lab. Med. 117, 196–198 (1993).
Schaffler, M. B., Cheung, W. Y., Majeska, R. & Kennedy, O. Osteocytes: master orchestrators of bone. Calcif. Tissue Int. 94, 5–24 (2014).
Kennedy, O. D. et al. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone 50, 1115–1122 (2012).
Kennedy, O. D., Laudier, D. M., Majeska, R. J., Sun, H. B. & Schaffler, M. B. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone 64, 132–137 (2014).
Schaffler, M. B. & Kennedy, O. D. Osteocyte signaling in bone. Curr. Osteoporos Rep. 10, 118–125 (2012).
Bancroft, L. W., Peterson, J. J. & Kransdorf, M. J. Cysts, geodes, and erosions. Radiol. Clin. North Am. 42, 73–87 (2004).
Carrino, J. A., Blum, J., Parellada, J. A., Schweitzer, M. E. & Morrison, W. B. MRI of bone marrow edema-like signal in the pathogenesis of subchondral cysts. Osteoarthritis Cartilage 14, 1081–1085 (2006).
Landells, J. W. The bone cysts of osteoarthritis. J. Bone Joint Surg. Br. 35-B, 643–649 (1953).
Rhaney, K. & Lamb, D. W. The cysts of osteoarthritis of the hip; a radiological and pathological study. J. Bone Joint Surg. Br. 37-B, 663–675 (1955).
Crema, M. D. et al. Subchondral cystlike lesions develop longitudinally in areas of bone marrow edema-like lesions in patients with or at risk for knee osteoarthritis: detection with MR imaging—the MOST study. Radiology 256, 855–862 (2010).
Crema, M. D. et al. Contrast-enhanced MRI of subchondral cysts in patients with or at risk for knee osteoarthritis: the MOST study. Eur. J. Radiol 75, e92–e96 (2010).
Raynauld, J. P. et al. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann. Rheum. Dis. 67, 683–688 (2008).
Tanamas, S. K. et al. The association between subchondral bone cysts and tibial cartilage volume and risk of joint replacement in people with knee osteoarthritis: a longitudinal study. Arthritis Res. Ther. 12, R58 (2010).
Wluka, A. E. et al. Bone marrow lesions can be subtyped into groups with different clinical outcomes using two magnetic resonance imaging (MRI) sequences. Arthritis Res. Ther. 17, 270 (2015).
Guyton, G. P. & Brand, R. A. Apparent spontaneous joint restoration in hip osteoarthritis. Clin. Orthop. Relat. Res. 404, 302–307 (2002).
Messent, E. A., Ward, R. J., Tonkin, C. J. & Buckland-Wright, C. Osteophytes, juxta-articular radiolucencies and cancellous bone changes in the proximal tibia of patients with knee osteoarthritis. Osteoarthritis Cartilage 15, 179–186 (2007).
van der Kraan, P. M. & van den Berg, W. B. Osteophytes: relevance and biology. Osteoarthritis Cartilage 15, 237–244 (2007).
Pottenger, L. A., Phillips, F. M. & Draganich, L. F. The effect of marginal osteophytes on reduction of varus-valgus instability in osteoarthritic knees. Arthritis Rheum. 33, 853–858 (1990).
Felson, D. T. et al. Osteophytes and progression of knee osteoarthritis. Rheumatology (Oxford) 44, 100–104 (2005).
Scharstuhl, A. et al. Inhibition of endogenous TGF-β during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J. Immunol. 169, 507–514 (2002).
Scharstuhl, A., Vitters, E. L., van der Kraan, P. M. & van den Berg, W. B. Reduction of osteophyte formation and synovial thickening by adenoviral overexpression of transforming growth factor β/bone morphogenetic protein inhibitors during experimental osteoarthritis. Arthritis Rheum. 48, 3442–3451 (2003).
van Beuningen, H. M., Glansbeek, H. L., van der Kraan, P. M. & van den Berg, W. B. Differential effects of local application of BMP-2 or TGF-β 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage 6, 306–317 (1998).
van Beuningen, H. M., Glansbeek, H. L., van der Kraan, P. M. & van den Berg, W. B. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-β injections. Osteoarthritis Cartilage 8, 25–33 (2000).
Findlay, D. M. & Atkins, G. J. Osteoblast-chondrocyte interactions in osteoarthritis. Curr. Osteoporos. Rep. 12, 127–134 (2014).
Brower, t. d., Akahoshi, Y. & Orlic, P. The diffusion of dyes through articular cartilage in vivo. J. Bone Joint Surg. 44, 456–453 (1962).
Maroudas, A., Bullough, P., Swanson, S. A. & Freeman, M. A. The permeability of cartilage. J. Bone Joint Surg. 50, 166–177 (1968).
Radin, E. L. & Rose, R. M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin. Orthop. Relat. Res. 212, 34–40 (1986).
Huebner, J. L., Hanes, M. A., Beekman, B., TeKoppele, J. M. & Kraus, V. B. A comparative analysis of bone and cartilage metabolism in two strains of guinea-pig with varying degrees of naturally occurring osteoarthritis. Osteoarthritis Cartilage 10, 758–767 (2002).
Amin, A. K., Huntley, J. S., Simpson, A. H. & Hall, A. C. Chondrocyte survival in articular cartilage: the influence of subchondral bone in a bovine model. J. Bone Joint Surg. Br. 91, 691–699 (2009).
Sanchez, C. et al. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytesfrom the sclerotic zones of osteoarthritic. Osteoarthritis Cartilage 13, 988–997 (2005).
Sanchez, C. et al. Phenotypic characterization of osteoblasts from the sclerotic zones of osteoarthritic subchondral bone. Arthritis Rheum. 58, 442–455 (2008).
Zhang, R. et al. Gene expression analysis of subchondral bone in early experimental osteoarthritis by microarray. PLoS ONE 7, e32356 (2012).
Chou, C. H. et al. Genome-wide expression profiles of subchondral bone in osteoarthritis. Arthritis Res. Ther. 15, R190 (2013).
Hilal, G., Martel-Pelletier, J., Pelletier, J. P., Ranger, P. & Lajeunesse, D. Osteoblast-like cells from human subchondral osteoarthritic bone demonstrate an altered phenotype in vitro: possible role in subchondral bone sclerosis. Arthritis Rheum. 41, 891–899 (1998).
Lotz, M. K. & Kraus, V. B. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res. Ther. 12, 211 (2010).
Martel-Pelletier, J., Wildi, L. M. & Pelletier, J. P. Future therapeutics for osteoarthritis. Bone 51, 297–311 (2012).
Mobasheri, A. The future of osteoarthritis therapeutics: emerging biological therapy. Curr. Rheumatol. Rep. 15, 385 (2013).
Mobasheri, A. The future of osteoarthritis therapeutics: targeted pharmacological therapy. Curr. Rheumatol. Rep. 15, 364 (2013).
Goldring, M. B. & Berenbaum, F. Emerging targets in osteoarthritis therapy. Curr. Opin. Pharmacol. 22, 51–63 (2015).
Goldring, S. R. Needs and opportunities in the assessment and treatment of osteoarthritis of the knee and hip: the view of the rheumatologist. J. Bone Joint Surg. Am. 91 (Suppl. 1), 4–6, (2009).
Karsdal, M. A. et al. The coupling of bone and cartilage turnover in osteoarthritis: opportunities for bone antiresorptives and anabolics as potential treatments? Ann. Rheum. Dis. 73, 336–348 (2014).
Roubille, C., Pelletier, J. P. & Martel-Pelletier, J. New and emerging treatments for osteoarthritis management: will the dream come true with personalized medicine? Expert Opin. Pharmacother. 14, 2059–2077 (2013).
Little, C. B. & Hunter, D. J. Post-traumatic osteoarthritis: from mouse models to clinical trials. Nat. Rev. Rheumatol. 9, 485–497 (2013).
Spector, T. D. et al. Effect of risedronate on joint structure and symptoms of knee osteoarthritis: results of the BRISK randomized, controlled trial [ISRCTN01928173]. Arthritis Res. Ther. 7, R625–R633 (2005).
Bingham, C. O., 3rd et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 54, 3494–3507 (2006).
Garnero, P. et al. Relationships between biochemical markers of bone and cartilage degradation with radiological progression in patients with knee osteoarthritis receiving risedronate: the Knee Osteoarthritis Structural Arthritis randomized clinical trial. Osteoarthritis Cartilage 16, 660–666 (2008).
Laslett, L. L. et al. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann. Rheum. Dis. 71, 1322–1328 (2012).
Karsdal, M. A. et al. Treatment of symptomatic knee osteoarthritis with oral salmon calcitonin: results from two phase 3 trials. Osteoarthritis Cartilage 23, 532–543 (2015).
Matthews, G. L. & Hunter, D. J. Emerging drugs for osteoarthritis. Expert Opin. Emerg. Drugs 16, 479–491 (2011).
Hawker, G. A. & Stanaitis, I. Osteoarthritis year in review 2014: clinical. Osteoarthritis Cartilage 22, 1953–1957 (2014).
Bay-Jensen, A. C. et al. Osteoarthritis year in review 2015: soluble biomarkers and the BIPED criteria. Osteoarthritis Cartilage 24, 9–20 (2016).
Kraus, V. B. et al. OARSI Clinical Trials Recommendations: Soluble biomarker assessments in clinical trials in osteoarthritis. Osteoarthritis Cartilage 23, 686–697 (2015).
Eckstein, F., Kwoh, C. K., Link, T. M. & OAI Investigators Imaging research results from the osteoarthritis initiative (OAI): a review and lessons learned 10 years after start of enrolment. Ann. Rheum. Dis. 73, 1289–1300 (2014).
Wright, T. Biomechanical factors in osteoarthritis: the effects of joint instability. HSS J. 8, 15–17 (2012).
Andriacchi, T. P., Favre, J., Erhart-Hledik, J. C. & Chu, C. R. A systems view of risk factors for knee osteoarthritis reveals insights into the pathogenesis of the disease. Ann. Biomed. Eng. 43, 376–387 (2015).
Courties, A., Gualillo, O., Berenbaum, F. & Sellam, J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage 23, 1955–1965 (2015).
Ko, F. C. et al. Progressive cell-mediated changes in articular cartilage and bone in mice are initiated by a single session of controlled cyclic compressive loading. J. Orthop. Res. http://dx.doi.org/10.1002/jor.23204 (2016).
Acknowledgements
The authors' work is supported by NIH grants R01-AG022021 (to M.B.G.) and RC4-AR060546 (to M.B.G.).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article and contributed to discussion of content, writing the article, and reviewing and editing the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Goldring, S., Goldring, M. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage–bone crosstalk. Nat Rev Rheumatol 12, 632–644 (2016). https://doi.org/10.1038/nrrheum.2016.148
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.148
This article is cited by
-
Glaucocalyxin A delays the progression of OA by inhibiting NF-κB and MAPK signaling pathways
Journal of Orthopaedic Surgery and Research (2024)
-
Nerve growth factor receptor limits inflammation to promote remodeling and repair of osteoarthritic joints
Nature Communications (2024)
-
Potential therapeutic strategies for osteoarthritis via CRISPR/Cas9 mediated gene editing
Reviews in Endocrine and Metabolic Disorders (2024)
-
Efficacy and safety of anti-interleukin-1 therapeutics in the treatment of knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials
Journal of Orthopaedic Surgery and Research (2023)
-
Human osteoarthritic articular cartilage stem cells suppress osteoclasts and improve subchondral bone remodeling in experimental knee osteoarthritis partially by releasing TNFAIP3
Stem Cell Research & Therapy (2023)