Abstract
That gut and joint inflammation are linked in spondyloarthritis (SpA) has been recognized for almost three decades. Intriguingly, microscopic gut inflammation, which occurs frequently in patients with SpA, is an important risk factor for clinically overt Crohn's disease and ankylosing spondylitis. This Review describes current insights into the underlying mechanisms that lead to chronic gut inflammation in patients with SpA. We propose that the development of chronic bowel inflammation in these individuals occurs through a transition phase, in which inflammation evolves from an acute into a chronic state. Our transition model implies that different cell types are involved at different stages during disease progression, with stromal cells having an important role in chronicity. In addition, deficient regulatory feedback mechanisms or genetically determined alterations in antigen presentation, endoplasmic reticulum stress, autophagy or cytokine signaling might also favor a transition from self-limiting acute inflammation to chronic inflammation. We anticipate that this transition phase might be an important window for therapeutic intervention.
Key Points
-
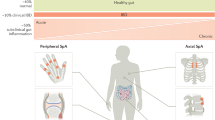
Microscopic gut inflammation occurs in approximately two-thirds of patients with spondyloarthritis
-
Chronic inflammatory lesions are an important risk factor for the development of Crohn's disease and ankylosing spondylitis, therefore indicating a potential link between gut and joint inflammation
-
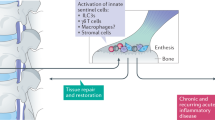
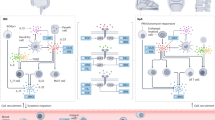
The transition of acute inflammatory lesions into chronic inflammatory lesions is influenced by deficient feedback of regulatory T cells
-
Mesenchymal cell types are important effector cells in the chronic phase of disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Dougados, M. & Baeten, D. Spondyloarthritis. Lancet 377, 2127–2137 (2011).
Sieper, J., Rudwaleit, M., Khan, M. A. & Braun, J. Concepts and epidemiology of spondyloarthritis. Best Pract. Res. Clin. Rheumatol. 20, 401–417 (2006).
van Tubergen, A. & Weber, U. Diagnosis and classification in spondyloarthritis: identifying a chameleon. Nat. Rev. Rheumatol. http://dx.doi.org/10.1038/nrrheum.2012.33
Elewaut, D. & Matucci-Cerinic, M. Treatment of ankylosing spondylitis and extra-articular manifestations in everyday rheumatology practice. Rheumatology (Oxford) 48, 1029–1035 (2009).
Sandborn, W. J. et al. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 121, 1088–1094 (2001).
de Vlam, K. et al. Spondyloarthropathy is underestimated in inflammatory bowel disease: prevalence and HLA association. J. Rheumatol. 27, 2860–2865 (2000).
Mielants, H. et al. The evolution of spondylarthropathies in relation to gut histology. II. Histological aspects. J. Rheumatol. 22, 2273–2278 (1995).
Altomonte, L. et al. Clinically silent inflammatory gut lesions in undifferentiated spondyloarthropathies. Clin. Rheumatol. 13, 565–570 (1994).
Leirisalo-Repo, M., Turunen, U., Stenman, S., Helenius, P. & Seppälä, K. High frequency of silent inflammatory bowel disease in spondylarthropathy. Arthritis Rheum. 37, 23–31 (1994).
Simenon, G., Van Gossum, A., Adler, M., Rickaert, F. & Appelboom, T. Macroscopic and microscopic gut lesions in seronegative spondyloarthropathies. J. Rheumatol. 17, 1491–1494 (1990).
Mielants, H., Veys, E., Cuvelier, C. & De Vos, M. Course of gut inflammation in spondylarthropathies and therapeutic consequences. Baillieres Clin. Rheumatol. 10, 147–164 (1996).
Schatteman, L. et al. Gut inflammation in psoriatic arthritis: a prospective ileocolonoscopic study. J. Rheumatol. 22, 680–683 (1995).
Mielants, H. et al. Gut inflammation in children with late onset pauciarticular juvenile chronic arthritis and evolution to adult spondyloarthropathy—a prospective study. J. Rheumatol. 20, 1567–1572 (1993).
Jacques, P. & Elewaut, D. Joint expedition: linking gut inflammation to arthritis. Mucosal Immunol. 1, 364–371 (2008).
Mielants, H. et al. The evolution of spondyloarthropathies in relation to gut histology. III. Relation between gut and joint. J. Rheumatol. 22, 2279–2284 (1995).
Cuvelier, C. et al. Histopathology of intestinal inflammation related to reactive arthritis. Gut 28, 394–401 (1987).
Mielants, H. et al. Gut inflammation in the spondyloarthropathies: clinical, radiologic, biologic and genetic features in relation to the type of histology. A prospective study. J. Rheumatol. 18, 1542–1551 (1991).
Reveille, J. D. Genetics of spondyloarthritis—beyond the MHC. Nat. Rev. Rheumatol. http://dx.doi.org/10.1038/nrrheum.2012.41
Thjodleifsson, B., Geirsson, A. J., Björnsson, S. & Bjarnason, I. A common genetic background for inflammatory bowel disease and ankylosing spondylitis: a genealogic study in Iceland. Arthritis Rheum. 56, 2633–2639 (2007).
Reveille, J. D. et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat. Genet. 42, 123–127 (2010).
Abraham, C. & Cho, J. Inflammatory bowel disease. N. Engl. J. Med. 361, 2066–2078 (2009).
Cho, J. H. & Brant, S. R. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology 140, 1704–1721 (2011).
Duerr, R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Burton, P. R. et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 (2007).
Franke, A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 42, 1118–1125 (2010).
Strange, A. et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat. Genet. 42, 985–990 (2010).
Yan, J. B. et al. In vivo role of ER-associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J. Exp. Med. 203, 647–659 (2006).
Cui, X. L. et al. Identification of ARTS-1 as a novel TNFR1-binding protein that promotes TNFR1 ectodomain shedding. J. Clin. Invest. 110, 515–526 (2002).
Reveille, J. D. The genetic basis of spondyloarthritis. Ann. Rheum. Dis. 70 (Suppl. 1), i44–i50 (2011).
Evans, D. M. et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 43, 761–767 (2011).
Danoy, P. et al. Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn's disease. PLoS Genet. 6, e1001195 (2010).
Baraliakos, X. et al. Interleukin-17A blockade with secukinumab reduces spinal inflammation in patients with ankylosing spondylitis as early as week 6, as detected by magnetic resonance imaging [abstract]. Arthritis Rheum. 63, 2486D (2011).
Hueber, W. et al. Inhibition of IL-17A by secukinumab is ineffective for Crohn's disease (CD). J. Crohns Colitis 5, S6–S7 (2011).
Leonardi, C. L. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371, 1665–1674 (2008).
Gottlieb, A. et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 373, 633–640 (2009).
Sandborn, W. J. et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterology 135, 1130–1141 (2008).
Sieper, J. Developments in therapy for spondyloarthritis. Nat. Rev. Rheumatol. http://dx.doi.org/10.1038/nrrheum.2012.40
Chen, C., Zhang, X. & Wang, Y. Analysis of JAK2 and STAT3 polymorphisms in patients with ankylosing spondylitis in Chinese Han population. Clin. Immunol. 136, 442–446 (2010).
Zhernakova, A. et al. Genetic analysis of innate immunity in Crohn's disease and ulcerative colitis identifies two susceptibility loci harboring CARD9 and IL18RAP. Am. J. Hum. Genet. 82, 1202–1210 (2008).
Pointon, J. J. et al. Elucidating the chromosome 9 association with AS; CARD9 is a candidate gene. Genes Immun. 11, 490–496 (2010).
Kugathasan, S. et al. Loci on 20q13 and 21q22 are associated with pediatric-onset inflammatory bowel disease. Nat. Genet. 40, 1211–1215 (2008).
Laukens, D. et al. CARD15 gene polymorphisms in patients with spondyloarthropathies identify a specific phenotype previously related to Crohn's disease. Ann. Rheum. Dis. 64, 930–935 (2005).
Vermeulen, N., Vermeire, S., Rutgeerts, P. & Bossuyt, X. Serological markers in inflammatory bowel disease. Immuno Analyse et Biologie Spécialisée 23, 358–367 (2008).
Hoffman, I. E. et al. Anti-Saccharomyces cerevisiae IgA antibodies are raised in ankylosing spondylitis and undifferentiated spondyloarthropathy. Ann. Rheum. Dis. 62, 455–459 (2003).
de Vries, M. et al. pANCA, ASCA, and OmpC antibodies in patients with ankylosing spondylitis without inflammatory bowel disease. J. Rheumatol. 37, 2340–2344 (2010).
Foell, D., Wittkowski, H. & Roth, J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut 58, 859–868 (2009).
Mundwiler, M. L. et al. Inflammatory bowel disease serologies in ankylosing spondylitis patients: a pilot study. Arthritis Res. Ther. 11, R177 (2009).
Klingberg, E., Carlsten, H., Hilme, E., Hedberg, M. & Forsblad-d'Elia, H. Calprotectin in ankylosing spondylitis—frequently elevated in feces, but normal in serum. Scand. J. Gastroenterol. http://dx.doi.org/10.3109/00365521.2011.648953.
Bjarnason, I. et al. Subclinical intestinal inflammation and sacroiliac changes in relatives of patients with ankylosing spondylitis. Gastroenterology 125, 1598–1605 (2003).
Sartor, R. B. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 3, 390–407 (2006).
Ott, S. J. et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 53, 685–693 (2004).
Frank, D. N. et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl Acad. Sci. USA 104, 13780–13785 (2007).
Friswell, M., Campbell, B. & Rhodes, J. The role of bacteria in the pathogenesis of inflammatory bowel disease. Gut Liver 4, 295–306 (2010).
Kang, S. et al. Dysbiosis of fecal microbiota in Crohn's disease patients as revealed by a custom phylogenetic microarray. Inflamm. Bowel Dis. 16, 2034–2042 (2010).
Tannock, G. W. The bowel microbiota and inflammatory bowel diseases. Int. J. Inflam. 2010, 954051 (2010).
Cebra, J. J., Periwal, S. B., Lee, G., Lee, F. & Shroff, K. E. Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev. Immunol. 6, 13–18 (1998).
Glaister, J. R. Factors affecting the lymphoid cells in the small intestinal epithelium of the mouse. Int. Arch. Allergy Appl. Immunol. 45, 719–730 (1973).
Taurog, J. D. et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 180, 2359–2364 (1994).
Rosenbaum, J. T. & Davey, M. P. Time for a gut check: evidence for the hypothesis that HLA-B27 predisposes to ankylosing spondylitis by altering the microbiome. Arthritis Rheum. 63, 3195–3198 (2011).
Benjamin, R. & Parham, P. Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol. Today 11, 137–142 (1990).
Kollnberger, S. et al. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 46, 2972–2982 (2002).
Wehkamp, J. et al. Paneth cell antimicrobial peptides: topographical distribution and quantification in human gastrointestinal tissues. FEBS Lett. 580, 5344–5350 (2006).
Wehkamp, J. et al. Reduced Paneth cell α-defensins in ileal Crohn's disease. Proc. Natl Acad. Sci. USA 102, 18129–18234 (2005).
Ciccia, F. et al. Over-expression of paneth cell-derived anti-microbial peptides in the gut of patients with ankylosing spondylitis and subclinical intestinal inflammation. Rheumatology (Oxford) 49, 2076–2083 (2010).
Ciccia, F. et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 60, 955–965 (2009).
Wu, L. & Van Kaer, L. Natural killer T cells and autoimmune disease. Curr. Mol. Med. 9, 4–14 (2009).
Wing, K. & Sakaguchi, S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 11, 7–13 (2010).
von Boehmer, H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 6, 338–344 (2005).
Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5, 405–417 (2007).
Maul, J. et al. Peripheral and intestinal regulatory CD4+ CD25high T cells in inflammatory bowel disease. Gastroenterology 128, 1868–1878 (2005).
Saruta, M. et al. Characterization of FOXP3+CD4+ regulatory T cells in Crohn's disease. Clin. Immunol. 125, 281–290 (2007).
Ciccia, F. et al. Expansion of intestinal CD4+CD25high TREG cells in patients with ankylosing spondylitis: a putative role for interleukin-10 in preventing intestinal TH17 response. Arthritis Rheum. 62, 3625–3634 (2010).
Boschetti, G. et al. Therapy with anti-TNFα antibody enhances number and function of FOXP3+ regulatory T cells in inflammatory bowel diseases. Inflamm. Bowel Dis. 17, 160–170 (2011).
Di Sabatino, A. et al. Peripheral regulatory T cells and serum transforming growth factor-β: relationship with clinical response to infliximab in Crohn's disease. Inflamm. Bowel Dis. 16, 1891–1897 (2010).
Cao, D., van Vollenhoven, R., Klareskog, L., Trollmo, C. & Malmström, V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res. Ther. 6, R335–R346 (2004).
Appel, H. et al. Synovial and peripheral blood CD4+FOXP3+ T cells in spondyloarthritis. J. Rheumatol. 38, 2445–2451 (2011).
Jacques, P. et al. Invariant natural killer T cells are natural regulators of murine spondylarthritis. Arthritis Rheum. 62, 988–999 (2010).
Baeten, D. et al. Comparative study of the synovial histology in rheumatoid arthritis, spondyloarthropathy, and osteoarthritis: influence of disease duration and activity. Ann. Rheum. Dis. 59, 945–953 (2000).
Baeten, D. et al. Impaired TH1 cytokine production in spondyloarthropathy is restored by anti-TNFα. Ann. Rheum. Dis. 60, 750–755 (2001).
Cañete, J. D. et al. Differential TH1/TH2 cytokine patterns in chronic arthritis: interferon γ is highly expressed in synovium of rheumatoid arthritis compared with seronegative spondyloarthropathies. Ann. Rheum. Dis. 59, 263–268 (2000).
Baeten, D. et al. Macrophages expressing the scavenger receptor CD163: a link between immune alterations of the gut and synovial inflammation in spondyloarthropathy. J. Pathol. 196, 343–350 (2002).
Melis, L. et al. Systemic levels of IL-23 are strongly associated with disease activity in rheumatoid arthritis but not spondyloarthritis. Ann. Rheum. Dis. 69, 618–623 (2010).
Melis, L. & Elewaut, D. Progress in spondyloarthritis. Immunopathogenesis of spondyloarthritis: which cells drive disease? Arthritis Res. Ther. 11, 233 (2009).
Rahman P. et al. Association of interleukin-23 receptor variants with ankylosing spondylitis. Arthritis Rheum. 58, 1020–1025 (2008).
DeLay, M. L. et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with TH17 activation in transgenic rats. Arthritis Rheum. 60, 2633–2643 (2009).
Appel, H. et al. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the TH17-mediated adaptive immune response. Arthritis Res. Ther. 13, R95 (2011).
Armaka, M. et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J. Exp. Med. 205, 331–337 (2008).
Roulis, M., Armaka, M., Manoloukos, M., Apostolaki, M. & Kollias, G. Intestinal epithelial cells as producers but not targets of chronic TNF suffice to cause murine Crohn-like pathology. Proc. Natl Acad. Sci. USA 108, 5396–5401 (2011).
Sugimoto K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008).
Wolk K. et al. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn's disease. J. Immunol. 178, 5973–5981 (2007).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature 473, 174–180 (2011).
Hindryckx, P. et al. Subclinical gut inflammation in spondyloarthritis is associated with a pro-angiogenic intestinal mucosal phenotype. Ann. Rheum. Dis. 70, 2044–2048 (2011).
Author information
Authors and Affiliations
Contributions
L. Van Praet and P. Jacques contributed equally to researching data for the article, discussing the content of the article and writing the article. F. Van den Bosch and D. Elewaut substantially contributed to discussions of the article content, wrote the article and reviewed and edited the manuscript before submission. D. Elewaut also researched some of the data for the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Van Praet, L., Jacques, P., Van den Bosch, F. et al. The transition of acute to chronic bowel inflammation in spondyloarthritis. Nat Rev Rheumatol 8, 288–295 (2012). https://doi.org/10.1038/nrrheum.2012.42
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2012.42
This article is cited by
-
Colonoscopy aspiration lavages for mucosal metataxonomic profiling of spondylarthritis-associated gastrointestinal tract alterations
Scientific Reports (2023)
-
Analyzing web searches for axial spondyloarthritis in Germany: a novel approach to exploring interests and unmet needs
Rheumatology International (2023)
-
Implementation of screening criteria for inflammatory bowel disease in patients with spondyloarthritis and its association with disease and endoscopic activity
Clinical Rheumatology (2023)
-
GM-CSF drives dysregulated hematopoietic stem cell activity and pathogenic extramedullary myelopoiesis in experimental spondyloarthritis
Nature Communications (2020)
-
Revisiting the gut–joint axis: links between gut inflammation and spondyloarthritis
Nature Reviews Rheumatology (2020)