Abstract
Osteoarthritis (OA) is the most prevalent joint disease, but neither preventive measures nor disease-modifying drugs are available and a continuing need exists for safe and effective symptom-modifying therapies. Clinical trials of candidate disease-modifying OA drugs in patients with established or advanced disease have not demonstrated their efficacy, but these failed trials have motivated investigation into the mechanisms that maintain joint health. The enhancement of such mechanisms could be a novel approach to reducing the risk of OA. Aging is one of the most important risk factors for OA; however, aging of joint cartilage is a process that is distinct from the subsequent cartilage changes that develop following the onset of OA. This Review focuses on the mechanisms that maintain cell and tissue homeostasis, and how these mechanisms fail during the aging process. Autophagy is a cellular homeostasis mechanism for the removal of dysfunctional organelles and macromolecules. Defective autophagy is involved in the pathogenesis of aging-related diseases and recent observations indicate that this process is compromised in aging cartilage. Augmentation of homeostasis mechanisms is discussed as a novel avenue to delay joint aging and reduce OA risk.
Key Points
-
In clinical trials in patients with established or advanced osteoarthritis (OA), candidate disease-modifying drugs have failed to show efficacy
-
Aging represents one of the main risk factors for OA, and pharmacological approaches that aim to delay cartilage aging could reduce OA risk
-
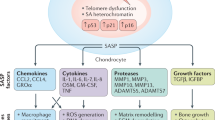
Failure of cellular homeostasis mechanisms is among the earliest events that precede cartilage cell death and extracellular matrix damage in the pathogenesis of OA
-
Autophagy is a central cellular homeostasis mechanism that is compromised in aging cartilage
-
Approaches to enhance autophagy and other homeostasis mechanisms might protect against aging-related cell dysfunction, and could be effective in reducing the risk for aging-related degenerative diseases such as OA
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lawrence, R. C. et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 58, 26–35 (2008).
Kim, S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997–2004. Arthritis Rheum. 59, 481–488 (2008).
Mahomed, N. N. et al. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J. Bone Joint Surg. Am. 85-A, 27–32 (2003).
Guccione, A. A. et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am. J. Public Health 84, 351–358 (1994).
Campbell, A. J., Borrie, M. J. & Spears, G. F. Risk factors for falls in a community-based prospective study of people 70 years and older. J. Gerontol. 44, M112–M117 (1989).
Zhang, W. et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 18, 476–499 (2010).
Hellio Le Graverand-Gastineau, M. P. OA clinical trials: current targets and trials for, OA. Choosing molecular targets: what have we learned and where we are headed? Osteoarthritis Cartilage 17, 1393–1401 (2009).
Herman, C. J., Allen, P., Hunt, W. C., Prasad, A. & Brady, T. J. Use of complementary therapies among primary care clinic patients with arthritis. Prev. Chronic Dis. 1, A12 (2004).
Ramsey, S. D., Spencer, A. C., Topolski, T. D., Belza, B. & Patrick, D. L. Use of alternative therapies by older adults with osteoarthritis. Arthritis Rheum. 45, 222–227 (2001).
Loeser, R. F. Molecular mechanisms of cartilage destruction in osteoarthritis. J. Musculoskelet. Neuronal Interact. 8, 303–306 (2008).
Felson, D. T. Developments in the clinical understanding of osteoarthritis. Arthritis Res. Ther. 11, 203 (2009).
Valdes, A. M. & Spector, T. D. The genetic epidemiology of osteoarthritis. Curr. Opin. Rheumatol. 22, 139–143 (2010).
Goldring, S. R. & Goldring, M. B. Bone and cartilage in osteoarthritis: is what's best for one good or bad for the other? Arthritis Res. Ther. 12, 143 (2010).
Scanzello, C. R. et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: Molecular characterization and relationship with symptoms. Arthritis Rheum. 63, 391–400 (2011).
Lohmander, L. S., Englund, P. M., Dahl, L. L. & Roos, E. M. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am. J. Sports Med. 35, 1756–1769 (2007).
Pelletier, J. P. & Martel-Pelletier, J. DMOAD developments: present and future. Bull. NYU Hosp. Jt Dis. 65, 242–248 (2007).
Matthews, G. L. & Hunter, D. J. Emerging drugs for osteoarthritis. Expert Opin. Emerg. Drugs doi:10.1517/14728214.2011.576670.
Miller, K. L. & Clegg, D. O. Glucosamine and chondroitin sulfate. Rheum. Dis. Clin. North Am. 37, 103–118 (2011).
Frech, T. M. & Clegg, D. O. The utility of nutraceuticals in the treatment of osteoarthritis. Curr. Rheumatol. Rep. 9, 25–30 (2007).
Le Graverand-Gastineau, M. P. Disease modifying osteoarthritis drugs: facing development challenges and choosing molecular targets. Curr. Drug Targets 11, 528–535 (2010).
Aigner, T., Rose, J., Martin, J. & Buckwalter, J. Aging theories of primary osteoarthritis: from epidemiology to molecular biology. Rejuvenation Res. 7, 134–145 (2004).
Loeser, R. F. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage 17, 971–979 (2009).
Horton, W. E. Jr, Bennion, P. & Yang, L. Cellular, molecular, and matrix changes in cartilage during aging and osteoarthritis. J. Musculoskelet. Neuronal Interact. 6, 379–381 (2006).
Blaney Davidson, E. N., Scharstuhl, A., Vitters, E. L., van der Krann, P. M. & van den Berg, W. B. Reduced transforming growth factor-β signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res. Ther. 7, R1338–R1347 (2005).
Barbero, A. et al. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage 12, 476–484 (2004).
van der Krann, P. M. & van den Berg, W. B. Osteoarthritis in the context of ageing and evolution: Loss of chondrocyte differentiation block during ageing. Ageing Res. Rev. 7, 106–113 (2008).
Poole, A. R., Guilak, F. & Abramson, S. “Etiopathogenesis of osteoarthritis” in Osteoarthritis: Diagnosis and Medical/Surgical Management (eds Moskowitz, R. W., Altman, R. D., Hochberg, M. C., Buckwalter, J. A. & Goldberg, V. M) (Lippincott, Williams & Williams, Philadelphia, 2007).
Hollander, A. P. et al. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J. Clin. Invest. 96, 2859–2869 (1995).
Guilak, F., Ratcliffe, A., Lane, N., Rosenwasser, M. P. & Mow, V. C. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J. Orthop. Res. 12, 474–484 (1994).
Panula, H. E. et al. Articular cartilage superficial zone collagen birefringence reduced and cartilage thickness increased before surface fibrillation in experimental osteoarthritis. Ann. Rheum. Dis. 57, 237–245 (1998).
Tchetina, E. V., Squires, G. & Poole, A. R. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J. Rheumatol. 32, 876–886 (2005).
Saarakkala, S. et al. Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis Cartilage 18, 73–81 (2010).
Temple, M. M. et al. Age- and site-associated biomechanical weakening of human articular cartilage of the femoral condyle. Osteoarthritis Cartilage 15, 1042–1052 (2007).
Temple-Wong, M. M. et al. Biomechanical, structural, and biochemical indices of degenerative and osteoarthritic deterioration of adult human articular cartilage of the femoral condyle. Osteoarthritis Cartilage 17, 1469–1476 (2009).
Carter, D. R. et al. The mechanobiology of articular cartilage development and degeneration. Clin. Orthop. Relat. Res. 427 (suppl.), S69–S77 (2004).
Darling, E. M., Hu, J. C. & Athanasiou, K. A. Zonal and topographical differences in articular cartilage gene expression. J. Orthop. Res. 22, 1182–1187 (2004).
Youn, I., Choi, J. B., Cao, L., Setton, L. A. & Guilak, F. Zonal variations in the three-dimensional morphology of the chondron measured in situ using confocal microscopy. Osteoarthritis Cartilage 14, 889–897 (2006).
Alsalameh, S., Amin, R., Gemba, T. & Lotz, M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 50, 1522–1532 (2004).
Dowthwaite, G. P. et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 117, 889–897 (2004).
Grogan, S. P., Miyaki, S., Asahara, H., D'Lima, D. D. & Lotz, M. K. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res. Ther. 11, R85 (2009).
Otsuki, S. et al. The effect of glycosaminoglycan loss on chondrocyte viability: a study on porcine cartilage explants. Arthritis Rheum. 58, 1076–1085 (2008).
Bae, W. C. et al. Indentation testing of human cartilage: sensitivity to articular surface degeneration. Arthritis Rheum. 48, 3382–3394 (2003).
Taniguchi, N. et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc. Natl Acad. Sci. USA 106, 1181–1186 (2009).
Taniguchi, N. et al. Chromatin protein HMGB2 regulates articular cartilage surface maintenance via beta-catenin pathway. Proc. Natl Acad. Sci. USA 106, 16817–16822 (2009).
Terman, A., Kurz, T., Navratil, M., Arriaga, E. A. & Brunk, U. T. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid. Redox Signal. 12, 503–535 (2010).
Morimoto, R. I. & Cuervo, A. M. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J. Gerontol. A Biol. Sci. Med. Sci. 64A, 167–170 (2009).
Fribley, A., Zhang, K. & Kaufman, R. J. Regulation of apoptosis by the unfolded protein response. Methods Mol. Biol. 559, 191–204 (2009).
Powers, E. T., Morimoto, R. I., Dillin, A., Kelly, J. W. & Balch, W. E. Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 78, 959–991 (2009).
Uchiyama, Y., Shibata, M., Koike, M., Yoshimura, K. & Sasaki, M. Autophagy—physiology and pathophysiology. Histochem. Cell Biol. 129, 407–420 (2008).
Ogata, M. et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell Biol. 26, 9220–9231 (2006).
Salminen, A., Kauppinen, A., Suuronen, T., Kaarniranta, K. & Ojala, J. ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology. J. Neuroinflammation 6, 41 (2009).
Austin, R. C. The unfolded protein response in health and disease. Antioxid. Redox Signal. 11, 2279–2287 (2009).
Kim, D. H., Davis, R. C., Furukawa, R. & Fechheimer, M. Autophagy contributes to degradation of Hirano bodies. Autophagy 5, 44–51 (2009).
Ong, D. S. & Kelly, J. W. Chemical and/or biological therapeutic strategies to ameliorate protein misfolding diseases. Curr. Opin. Cell Biol. 23, 231–238 (2010).
Mizushima, N. Physiological functions of autophagy. Curr. Top. Microbiol. Immunol. 335, 71–84 (2009).
Rubinsztein, D. C., Gestwicki, J. E., Murphy, L. O. & Klionsky, D. J. Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov. 6, 304–312 (2007).
Mizushima, N., Levine, B., Cuervo, A. M. & Klionsky, D. J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008).
Yu, L. et al. Autophagic programmed cell death by selective catalase degradation. Proc. Natl Acad. Sci. USA 103, 4952–4957 (2006).
Codogno, P. & Meijer, A. J. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 12 (Suppl. 2), 1509–1518 (2005).
Baehrecke, E. H. Autophagy: dual roles in life and death? Nat. Rev. Mol. Cell Biol. 6, 505–510 (2005).
Chan, E. Y. & Tooze, S. A. Evolution of Atg1 function and regulation. Autophagy 5, 758–765 (2009).
Furuya, N., Yu, J., Byfield, M., Pattingre, S. & Levine, B. The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Autophagy 1, 46–52 (2005).
Ohsumi, Y. & Mizushima, N. Two ubiquitin-like conjugation systems essential for autophagy. Semin. Cell Dev. Biol. 15, 231–236 (2004).
Mizushima, N. & Klionsky, D. J. Protein turnover via autophagy: implications for metabolism. Annu. Rev. Nutr. 27, 19–40 (2007).
Finkel, T. & Holbrook, N. J. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 (2000).
Stanfel, M. N., Shamieh, L. S., Kaeberlein, M. & Kennedy, B. K. The TOR pathway comes of age. Biochim. Biophys. Acta 1790, 1067–1074 (2009).
Bonawitz, N. D., Chatenay-Lapointe, M., Pan, Y. & Shadel, G. S. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 5, 265–277 (2007).
Hara, T. et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 (2006).
Goldring, M. B. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract. Res. Clin. Rheumatol. 20, 1003–1025 (2006).
Johansen, T. & Lamark, T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 7, 279–296 (2011).
Blanco, F. J., Rego, I. & Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 7, 161–169 (2011).
Cuervo, A. M. Autophagy and aging: keeping that old broom working. Trends Genet. 24, 604–612 (2008).
Carames, B., Taniguchi, N., Otsuki, S., Blanco, F. J. & Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 62, 791–801 (2010).
Cuervo, A. M. & Dice, J. F. Age-related decline in chaperone-mediated autophagy. J. Biol. Chem. 275, 31505–31513 (2000).
Martinez, A., Portero-Otin, M., Pamplona, R. & Ferrer, I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol. 20, 281–297 (2010).
Brunk, U. T. & Terman, A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 33, 611–619 (2002).
Lee, H. K., Lund, J. M., Ramanathan, B., Mizushima, N. & Iwasaki, A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 315, 1398–1401 (2007).
Raben, N. et al. Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease. Hum. Mol. Genet. 17, 3897–3908 (2008).
Vellai, T., Takacs-Vellai, K., Sass, M. & Klionsky, D. J. The regulation of aging: does autophagy underlie longevity? Trends Cell Biol. 19, 487–494 (2009).
Cuervo, A. M. et al. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy 1, 131–140 (2005).
Salminen, A. & Kaarniranta, K. Regulation of the aging process by autophagy. Trends Mol. Med. 15, 217–224 (2009).
Hadley, E. C., Lakatta, E. G., Morrison-Bogorad, M., Warner, H. R. & Hodes, R. J. The future of aging therapies. Cell 120, 557–567 (2005).
Masoro, E. J. Overview of caloric restriction and ageing. Mech. Ageing Dev. 126, 913–922 (2005).
Smith, G. K. et al. Lifelong diet restriction and radiographic evidence of osteoarthritis of the hip joint in dogs. J. Am. Vet. Med. Assoc. 229, 690–693 (2006).
Madeo, F., Tavernarakis, N. & Kroemer, G. Can autophagy promote longevity? Nat. Cell Biol. 12, 842–846 (2010).
Nadon, N. L. et al. Design of aging intervention studies: the NIA interventions testing program. Age (Dordr) 30, 187–199 (2008).
Strong, R. et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell 7, 641–650 (2008).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Miller, R. A. et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 66A, 191–201 (2011).
Sofroniadou, S. & Goldsmith, D. Mammalian target of rapamycin (mTOR) inhibitors: potential uses and a review of haematological adverse effects. Drug Saf. 34, 97–115 (2011).
Renna, M., Jimenez-Sanchez, M., Sarkar, S. & Rubinsztein, D. C. Chemical inducers of autophagy that enhance the clearance of mutant proteins in neurodegenerative diseases. J. Biol. Chem. 285, 11061–11067 (2010).
Sage, A. T. et al. Hexosamine biosynthesis pathway flux promotes endoplasmic reticulum stress, lipid accumulation, and inflammatory gene expression in hepatic cells. Am. J. Physiol. Endocrinol. Metab. 298, E499–E511 (2010).
Matthews, J. A., Belof, J. L., Acevedo-Duncan, M. & Potter, R. L. Glucosamine-induced increase in Akt phosphorylation corresponds to increased endoplasmic reticulum stress in astroglial cells. Mol. Cell Biochem. 298, 109–123 (2007).
Qiu, W., Su, Q., Rutledge, A. C., Zhang, J. & Adeli, K. Glucosamine-induced endoplasmic reticulum stress attenuates apolipoprotein B100 synthesis via PERK signaling. J. Lipid Res. 50, 1814–1823 (2009).
Shintani, T. et al. Glucosamine induces autophagy via an mTOR-independent pathway. Biochem. Biophys. Res. Commun. 391, 1775–1779 (2010).
Poole, R. et al. Recommendations for the use of preclinical models in the study and treatment of osteoarthritis. Osteoarthritis Cartilage 18 (Suppl. 3), S10–S16 (2010).
De Ceuninck, F., Sabatini, M. & Pastoureau, P. Recent progress toward biomarker identification in osteoarthritis. Drug Discov. Today 16, 443–449 (2011).
Acknowledgements
The writing of this manuscript was supported by NIH grants AG007996 and AR056026, and a grant from Cargill, Inc.
Author information
Authors and Affiliations
Contributions
B. Caramés and M. K. Lotz researched the data for the article, provided substantial contributions to discussions of the content, and contributed to writing the article and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M. K Lotz has acted as a consultant for and received grant/research support from Cargill, Inc. B. Caramés declares no competing interests.
Rights and permissions
About this article
Cite this article
Lotz, M., Caramés, B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol 7, 579–587 (2011). https://doi.org/10.1038/nrrheum.2011.109
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2011.109
This article is cited by
-
Oxymatrine protects articular chondrocytes from IL-1β-induced damage through autophagy activation via AKT/mTOR signaling pathway inhibition
Journal of Orthopaedic Surgery and Research (2024)
-
Saikosaponin D alleviates inflammatory response of osteoarthritis and mediates autophagy via elevating microRNA-199-3p to target transcription Factor-4
Journal of Orthopaedic Surgery and Research (2024)
-
Type I Collagen/Hyaluronic Acid Hydrogels as Delivery System for Adipose-Derived Stem Cells for Osteoarthritis Treatment
Regenerative Engineering and Translational Medicine (2024)
-
Engineering exosomes derived from subcutaneous fat MSCs specially promote cartilage repair as miR-199a-3p delivery vehicles in Osteoarthritis
Journal of Nanobiotechnology (2023)
-
Muti-factor analysis of sport activity level after high tibial osteotomy
Journal of Orthopaedic Surgery and Research (2023)