Abstract
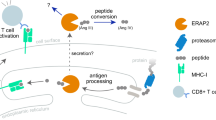
Endoplasmic reticulum aminopeptidase 1 (ERAP1) and the closely related ERAP2 are involved in the final trimming of peptides within the endoplasmic reticulum for presentation by major histocompatibility complex (MHC) class I molecules. ERAP1 was found to be associated with ankylosing spondylitis (AS) in a genome-wide association study of nonsynonymous single nucleotide polymorphisms, and this association has been confirmed in several studies. An ERAP1/ERAP2 haplotype has also been reported to be associated with familial AS. ERAP1 and ERAP2 could carry out several potential roles in the pathogenesis of AS. ERAP1-deficient mice show a considerable alteration in the level and repertoire of peptides presented by MHC class I molecules. Furthermore, ERAP1 has been shown to be involved in shedding cytokine receptors. Both of these functions require further analysis to better understand the exact role of ERAP1 in AS.
Key Points
-
An association between the genes encoding endoplasmic reticulum aminopeptidase (ERAP)1 and ERAP2 and ankylosing spondylitis (AS) has been identified
-
ERAP1 and ERAP2 act in concert to trim and present peptides on major histocompatibility complex class I molecules within the endoplasmic reticulum
-
ERAP1 is also known to aid in the shedding of membrane-bound cytokine receptors
-
Preliminary data suggest that the peptide-trimming functions of ERAP1 and ERAP2 might be important in the pathogenesis of AS
-
Determining the crystallographic structure of ERAP1 should help greatly in the study of the function of this molecule
-
Large cohort studies looking at the clinical relevance of the association between ERAP1 and AS could shed more light on this novel and promising lead
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Brown, M. A. et al. Susceptibility to ankylosing spondylitis in twins: the role of genes, HLA, and the environment. Arthritis Rheum. 40, 1823–1828 (1997).
Jarvinen, P. Occurrence of ankylosing spondylitis in a nationwide series of twins. Arthritis Rheum. 38, 381–383 (1995).
Pedersen, O. B. et al. Ankylosing spondylitis in Danish and Norwegian twins: occurrence and the relative importance of genetic vs environmental effectors in disease causation. Scand. J. Rheumatol. 37, 120–126 (2008).
Brewerton, D. A. et al. Ankylosing spondylitis and HL-A 27. Lancet 1, 904–907 (1973).
Brown, M. A., Laval, S. H., Brophy, S. & Calin, A. Recurrence risk modelling of the genetic susceptibility to ankylosing spondylitis. Ann. Rheum. Dis. 59, 883–886 (2000).
Laval, S. H. et al. Whole-genome screening in ankylosing spondylitis: evidence of non-MHC genetic-susceptibility loci. Am. J. Hum. Genet. 68, 918–926 (2001).
Wellcome Trust Case Control Consortium et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 (2007).
Maksymowych, W. P. et al. Association of a specific ERAP1/ARTS1 haplotype with disease susceptibility in ankylosing spondylitis. Arthritis Rheum. 60, 1317–1323 (2009).
Davidson, S. I. et al. Association of ERAP1, but not IL23R, with ankylosing spondylitis in a Han Chinese population. Arthritis Rheum. 60, 3263–3268 (2009).
Choi, C. B. et al. ARTS1 polymorphisms are associated with ankylosing spondylitis in Koreans. Ann. Rheum. Dis. 69, 582–584 (2010).
Pimentel-Santos, F. M. et al. Association of IL23R and ERAP1 genes with ankylosing spondylitis in a Portuguese population. Clin. Exp. Rheumatol. 27, 800–806 (2009).
Harvey, D. et al. Investigating the genetic association between ERAP1 and ankylosing spondylitis. Hum. Mol. Genet. 18, 4204–4212 (2009).
Tsui, F. W. et al. Association of an ERAP1 ERAP2 haplotype with familial ankylosing spondylitis. Ann. Rheum. Dis. 69, 733–736 (2010).
Tanioka, T. et al. Human leukocyte-derived arginine aminopeptidase. The third member of the oxytocinase subfamily of aminopeptidases. J. Biol. Chem. 278, 32275–32283 (2003).
The Ensembl website [online], (2010).
Chang, S. C., Momburg, F., Bhutani, N. & Goldberg, A. L. The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc. Natl Acad. Sci. USA 102, 17107–17112 (2005).
Evnouchidou, I. et al. The internal sequence of the peptide-substrate determines its N-terminus trimming by ERAP1. PLoS ONE 3, e3658 (2008).
Neisig, A. et al. Major differences in transporter associated with antigen presentation (TAP)-dependent translocation of MHC class I-presentable peptides and the effect of flanking sequences. J. Immunol. 154, 1273–1279 (1995).
Blanchard, N. & Shastri, N. Coping with loss of perfection in the MHC class I peptide repertoire. Curr. Opin. Immunol. 20, 82–88 (2008).
Saric, T. et al. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat. Immunol. 3, 1169–1176 (2002).
Dixon, A. L. et al. A genome-wide association study of global gene expression. Nat. Genet. 39, 1202–1207 (2007).
Serwold, T., Gonzalez, F., Kim, J., Jacob, R. & Shastri, N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature 419, 480–483 (2002).
Hammer, G. E., Gonzalez, F., James, E., Nolla, H. & Shastri, N. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat. Immunol. 8, 101–108 (2007).
York, I. A. et al. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8–9 residues. Nat. Immunol. 3, 1177–1184 (2002).
Kanaseki, T. & Shastri, N. Endoplasmic reticulum aminopeptidase associated with antigen processing regulates quality of processed peptides presented by MHC class I molecules. J. Immunol. 181, 6275–6282 (2008).
Gibbs, R. A. et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 428, 493–521 (2004).
Saveanu, L. et al. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat. Immunol. 6, 689–697 (2005).
Haroon, N. et al. ERAP1 Q730E variants affect the HLA B27 free chain expression on monocytes of patients with ankylosing spondylitis [abstract #1448]. Arthritis Rheum. 60 (Suppl.), S541 (2009).
Turner, M. J., Delay, M. L., Bai, S., Klenk, E. & Colbert, R. A. HLA-B27 up-regulation causes accumulation of misfolded heavy chains and correlates with the magnitude of the unfolded protein response in transgenic rats: Implications for the pathogenesis of spondylarthritis-like disease. Arthritis Rheum. 56, 215–223 (2007).
Turner, M. J. et al. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J. Immunol. 175, 2438–2448 (2005).
DeLay, M. L. et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 60, 2633–2643 (2009).
Harris, M. R. et al. Interactions of HLA-B27 with the peptide loading complex as revealed by heavy chain mutations. Int. Immunol. 13, 1275–1282 (2001).
Fukazawa, T. et al. The effect of mutant beta 2-microglobulins on the conformation of HLA-B27 detected by antibody and by CTL. J. Immunol. 153, 3543–3550 (1994).
Allen, R. L. & Trowsdale, J. Recognition of classical and heavy chain forms of HLA-B27 by leukocyte receptors. Curr. Mol. Med. 4, 59–65 (2004).
Raine, T. et al. Consistent patterns of expression of HLA class I free heavy chains in healthy individuals and raised expression in spondyloarthropathy patients point to physiological and pathological roles. Rheumatology (Oxford) 45, 1338–1344 (2006).
Kollnberger, S. et al. Interaction of HLA-B27 homodimers with KIR3DL1 and KIR3DL2, unlike HLA-B27 heterotrimers, is independent of the sequence of bound peptide. Eur. J. Immunol. 37, 1313–1322 (2007).
Kollnberger, S. et al. HLA-B27 heavy chain homodimers are expressed in HLA-B27 transgenic rodent models of spondyloarthritis and are ligands for paired Ig-like receptors. J. Immunol. 173, 1699–1710 (2004).
Kollnberger, S. et al. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 46, 2972–2982 (2002).
Cui, X., Rouhani, F. N., Hawari, F. & Levine, S. J. Shedding of the type II IL-1 decoy receptor requires a multifunctional aminopeptidase, aminopeptidase regulator of TNF receptor type 1 shedding. J. Immunol. 171, 6814–6819 (2003).
Cui, X., Rouhani, F. N., Hawari, F. & Levine, S. J. An aminopeptidase, ARTS-1, is required for interleukin-6 receptor shedding. J. Biol. Chem. 278, 28677–28685 (2003).
Cui, X. et al. Identification of ARTS-1 as a novel TNFR1-binding protein that promotes TNFR1 ectodomain shedding. J. Clin. Invest. 110, 515–526 (2002).
Islam, A. et al. Extracellular TNFR1 release requires the calcium-dependent formation of a nucleobindin 2-ARTS-1 complex. J. Biol. Chem. 281, 6860–6873 (2006).
Adamik, B. et al. An association between RBMX, a heterogeneous nuclear ribonucleoprotein, and ARTS-1 regulates extracellular TNFR1 release. Biochem. Biophys. Res. Commun. 371, 505–509 (2008).
Layh-Schmitt, G. & Colbert, R. A. The interleukin-23/interleukin-17 axis in spondyloarthritis. Curr. Opin. Rheumatol. 20, 392–397 (2008).
Sims, A. M. et al. Prospective meta-analysis of interleukin 1 gene complex polymorphisms confirms associations with ankylosing spondylitis. Ann. Rheum. Dis. 67, 1305–1309 (2008).
Haroon, N., Tsui, F. W. L., Chiu, B. & Inman, R. D. Serum cytokine receptors in ankylosing spondylitis: relationship to inflammatory markers and endoplasmic reticulum aminopeptidase polymorphisms. J. Rheum. (in press).
Haroon, N., O'Shea, F. D., Rahman, P., Tsui, F. W. & Inman, R. D. ERAP1 R528 variants influence the radiological progression in ankylosing spondylitis [abstract #1445]. Arthritis Rheum. 60 (Suppl.), S540 (2009).
Colbert, R. A., DeLay, M. L., Layh-Schmitt, G. & Sowders, D. P. HLA-B27 misfolding and spondyloarthropathies. Adv. Exp. Med. Biol. 649, 217–234 (2009).
Khare, S. D., Hansen, J., Luthra, H. S. & David, C. S. HLA-B27 heavy chains contribute to spontaneous inflammatory disease in B27/human beta2-microglobulin (beta2m) double transgenic mice with disrupted mouse beta2m. J. Clin. Invest. 98, 2746–2755 (1996).
Tsai, W. C. et al. Free HLA class I heavy chain-carrying monocytes—a potential role in the pathogenesis of spondyloarthropathies. J. Rheumatol. 29, 966–972 (2002).
Tanigaki, N. et al. The peptide binding specificity of HLA-B27 subtypes. Immunogenetics 40, 192–198 (1994).
Fiorillo, M. T. et al. Susceptibility to ankylosing spondylitis correlates with the C-terminal residue of peptides presented by various HLA-B27 subtypes. Eur. J. Immunol. 27, 368–373 (1997).
Lahesmaa, R., Skurnik, M. & Toivanen, P. Molecular mimicry: any role in the pathogenesis of spondyloarthropathies? Immunol. Res. 12, 193–208 (1993).
Allen, R. L., Raine, T., Haude, A., Trowsdale, J. & Wilson, M. J. Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J. Immunol. 167, 5543–5547 (2001).
Taranta, A. et al. Genetic risk factors in typical haemolytic uraemic syndrome. Nephrol. Dial. Transplant. 24, 1851–1857 (2009).
Yamamoto, N. et al. Identification of 33 polymorphisms in the adipocyte-derived leucine aminopeptidase (ALAP) gene and possible association with hypertension. Hum. Mutat. 19, 251–257 (2002).
Fung, E. Y. et al. Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes Immun. 10, 188–191 (2009).
Johnson, M. P. et al. The ERAP2 gene is associated with preeclampsia in Australian and Norwegian populations. Hum. Genet. 126, 655–666 (2009).
Saito, S., Sakai, M., Sasaki, Y., Nakashima, A. & Shiozaki, A. Inadequate tolerance induction may induce pre-eclampsia. J. Reprod. Immunol. 76, 30–39 (2007).
Ponte, M. et al. Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor. Proc. Natl Acad. Sci. USA 96, 5674–5679 (1999).
Yan, W. H. et al. Possible roles of KIR2DL4 expression on uNK cells in human pregnancy. Am. J. Reprod. Immunol. 57, 233–242 (2007).
Shido, F. et al. Endoplasmic reticulum aminopeptidase-1 mediates leukemia inhibitory factor-induced cell surface human leukocyte antigen-G expression in JEG-3 choriocarcinoma cells. Endocrinology 147, 1780–1788 (2006).
Blanchard, N. et al. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat. Immunol. 9, 937–944 (2008).
Draenert, R. et al. Immune selection for altered antigen processing leads to cytotoxic T lymphocyte escape in chronic HIV-1 infection. J. Exp. Med. 199, 905–915 (2004).
Mehta, A. M. et al. Single nucleotide polymorphisms in antigen processing machinery component ERAP1 significantly associate with clinical outcome in cervical carcinoma. Genes Chromosomes Cancer 48, 410–418 (2009).
Mehta, A. M., Jordanova, E. S., Kenter, G. G., Ferrone, S. & Fleuren, G. J. Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol. Immunother. 57, 197–206 (2008).
Mehta, A. M. et al. Genetic variation of antigen processing machinery components and association with cervical carcinoma. Genes Chromosomes Cancer 46, 577–586 (2007).
Fruci, D. et al. Altered expression of endoplasmic reticulum aminopeptidases ERAP1 and ERAP2 in transformed non-lymphoid human tissues. J. Cell Physiol. 216, 742–749 (2008).
Fruci, D. et al. Expression of endoplasmic reticulum aminopeptidases in EBV-B cell lines from healthy donors and in leukemia/lymphoma, carcinoma, and melanoma cell lines. J. Immunol. 176, 4869–4879 (2006).
Yamada, Y., Ando, F. & Shimokata, H. Association of candidate gene polymorphisms with bone mineral density in community-dwelling Japanese women and men. Int. J. Mol. Med. 19, 791–801 (2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Haroon, N., Inman, R. Endoplasmic reticulum aminopeptidases: biology and pathogenic potential. Nat Rev Rheumatol 6, 461–467 (2010). https://doi.org/10.1038/nrrheum.2010.85
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.85
This article is cited by
-
Genetic association of ERAP1 and ERAP2 with eclampsia and preeclampsia in northeastern Brazilian women
Scientific Reports (2021)
-
Genetic association between TAP1 and TAP2 polymorphisms and ankylosing spondylitis: a systematic review and meta-analysis
Inflammation Research (2017)
-
ERAP1 rs30187 single nucleotide polymorphism does not confer disease susceptibility in North Indian children with enthesitis-related arthritis
Clinical Rheumatology (2017)
-
Genetic associations and functional characterization of M1 aminopeptidases and immune-mediated diseases
Genes & Immunity (2014)
-
Genetic Association with ERAP1 in Psoriasis Is Confined to Disease Onset after Puberty and Not Dependent on HLA-C*06
Journal of Investigative Dermatology (2013)