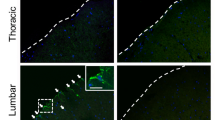
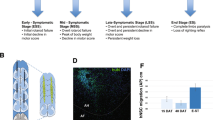
Abstract
Effective treatments are urgently needed for amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disease characterized by the loss of motor neurons. In 2009, the FDA approved the first phase I safety trial of direct intraspinal transplantation of neural stem cells into patients with ALS, which is currently in progress. Stem cell technologies represent a promising approach for treating ALS, but several issues must be addressed when translating promising experimental ALS therapies to patients. This article highlights the key research that supports the use of stem cells as a therapy for ALS, and discusses the rationale behind and approach to the phase I trial. Completion of the trial could pave the way for continued advances in stem cell therapy for ALS and other neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Borasio, G. & Miller, R. Clinical characteristics and management of ALS. Semin. Neurosci. 21, 155–166 (2001).
Miller, R. H. The promise of stem cells for neural repair. Brain Res. 1091, 258–264 (2006).
Rothstein, J. D. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann. Neurol. 65 (Suppl. 1), S3–S9 (2009).
Ilieva, H., Polymenidou, M. & Cleveland, D. W. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 187, 761–772 (2009).
Bonner, J. F., Blesch, A., Neuhuber, B. & Fischer, I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J. Neurosci. Res. 88, 1182–1192 (2010).
Silani, V., Calzarossa, C., Cova, L. & Ticozzi, N. Stem cells in amyotrophic lateral sclerosis: motor neuron protection or replacement? CNS Neurol. Disord. Drug Targets 9, 314–324 (2010).
Xu, L., Ryugo, D. K., Pongstaporn, T., Johe, K. & Koliatsos, V. E. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J. Comp. Neurol. 514, 297–309 (2009).
Yan, J. et al. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 4, e39 (2007).
Boucherie, C., Schafer, S., Lavand'homme, P., Maloteaux, J. M. & Hermans, E. Chimerization of astroglial population in the lumbar spinal cord after mesenchymal stem cell transplantation prolongs survival in a rat model of amyotrophic lateral sclerosis. J. Neurosci. Res. 87, 2034–2046 (2009).
Clement, A. M. et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302, 113–117 (2003).
Lepore, A. C. et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat. Neurosci. 11, 1294–301 (2008).
Fischer, L. R. & Glass, J. D. Axonal degeneration in motor neuron disease. Neurodegener. Dis. 4, 431–442 (2007).
Sakowski, S. A. et al. Neuroprotection using gene therapy to induce vascular endothelial growth factor-A expression. Gene Ther. 16, 1292–1299 (2009).
Sakowski, S. A., Schuyler, A. D. & Feldman, E. L. Insulin-like growth factor-I for the treatment of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 10, 63–73 (2009).
Sorenson, E. J. et al. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology 71, 1770–1775 (2008).
Suzuki, M. et al. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS ONE 2, e689 (2007).
Park, S. et al. Growth factor-expressing human neural progenitor cell grafts protect motor neurons but do not ameliorate motor performance and survival in ALS mice. Exp. Mol. Med. 41, 487–500 (2009).
Suzuki, M. et al. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol. Ther. 16, 2002–2010 (2008).
Xu, L. et al. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation 82, 865–875 (2006).
Yan, J. et al. Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells 24, 1976–1985 (2006).
Xu, L., Shen, P., Hazel, T., Johe, K. & Koliatsos, V. E. Dual transplantation of human neural stem cells into cervical and lumbar cord ameliorates motor neuron disease in SOD1 transgenic rats. Neurosci. Lett. 494, 222–226 (2011).
Lunn, J. S., Hefferan, M. P., Marsala, M. & Feldman, E. L. Stem cells: comprehensive treatments for amyotrophic lateral sclerosis in conjunction with growth factor delivery. Growth Factors 27, 133–140 (2009).
Lunn, J. S. et al. Stem cell technology for the study and treatment of motor neuron diseases. Regen. Med. 6, 201–213 (2011).
Tetzlaff, W. et al. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma 28, 1611–1682 (2011).
Mazzini, L. et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a Phase I clinical trial. Exp. Neurol. 223, 229–237 (2010).
Chen, L. et al. Short-term outcome of olfactory ensheathing cells transplantation for treatment of amyotrophic lateral sclerosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 21, 961–966 (2007).
Deda, H. et al. Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: a 1-year follow-up. Cytotherapy 11, 18–25 (2009).
Raore, B. et al. Cervical multilevel intraspinal stem cell therapy: assessment of surgical risks in Gottingen minipigs. Spine (Phila Pa 1976) 36, E164–E171 (2011).
Riley, J. P., Raore, B., Taub, J. S., Federici, T. & Boulis, N. M. Platform and cannula design improvements for spinal cord therapeutics delivery. Neurosurgery http://dx.doi.org/10.1227/NEU.0b013e3182195680.
Usvald, D. et al. Analysis of dosing regimen and reproducibility of intraspinal grafting of human spinal stem cells in immunosuppressed minipigs. Cell Transplant. 19, 1103–1122 (2010).
Hefferan, M. P., Johe, K., Feldman, E. L., Lunn, J. S. & Marsala, M. Optimization of immunosuppressive therapy for spinal grafting of human spinal stem cells in a rat model of ALS. Cell Transplant. http://dx.doi.org/10.3727/096368910X564553.
Guest, J., Benavides, F., Padgett, K., Mendez, E. & Tovar, D. Technical aspects of spinal cord injections for cell transplantation. Clinical and translational considerations. Brain Res. Bull. 84, 267–279 (2011).
Bretzner, F., Gilbert, F., Baylis, F. & Brownstone, R. M. Target populations for first-in-human embryonic stem cell research in spinal cord injury. Cell Stem Cell 8, 468–475 (2011).
Acknowledgements
We thank M. Marsala, M. Hefferan, J. Rile, B. Raore and J. Taub for their technical assistance with the preclinical studies. Preclinical studies were funded in part by the A. Alfred Taubman Medical Research Institute, the ALS Association and Neuralstem. N. Boulis invented the stem cell delivery device used in the phase I trial. The phase I trial is funded by Neuralstem, and we acknowledge the valuable input of K. Johe and the Data Safety Monitoring Board of the trial. We are grateful to the Emory ALS Center, patients and families for their participation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
N. M. Boulis invented the device utilized in the trial for the safe and accurate injection of stem cells into the human spinal cord. He received an inventor fee and retains rights to royalty payments on distribution of the device. Exclusive licensure for this technology has been purchased by NeuralStem. N. M. Boulis, T. Federici and J. D. Glass received funding from NeuralStem for the phase I trial. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Boulis, N., Federici, T., Glass, J. et al. Translational stem cell therapy for amyotrophic lateral sclerosis. Nat Rev Neurol 8, 172–176 (2012). https://doi.org/10.1038/nrneurol.2011.191
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2011.191
This article is cited by
-
A perspective on therapies for amyotrophic lateral sclerosis: can disease progression be curbed?
Translational Neurodegeneration (2021)
-
Targeted intraspinal injections to assess therapies in rodent models of neurological disorders
Nature Protocols (2019)
-
Ethical development of stem-cell-based interventions
Nature Medicine (2019)
-
Advances, Challenges, and Perspectives in Translational Stem Cell Therapy for Amyotrophic Lateral Sclerosis
Molecular Neurobiology (2019)
-
Endothelial and Astrocytic Support by Human Bone Marrow Stem Cell Grafts into Symptomatic ALS Mice towards Blood-Spinal Cord Barrier Repair
Scientific Reports (2017)