Key Points
-
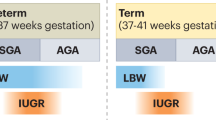
Low birth weight, prematurity, and small size for gestational age are associated with reduced nephron numbers and an increased risk of hypertension and kidney disease in later life
-
High birth weight and gestational exposure to maternal diabetes or obesity are important factors associated with an increased risk of hypertension and kidney disease in later life
-
Maternal nutrition before and during pregnancy impacts birth weight and risk of premature delivery; maternal nutrient deficiencies can affect the development of the fetal kidneys
-
Postnatal nutrition, acute kidney injury, and clinical events can further impact nephrogenesis soon after premature delivery
-
A rapid increase in weight and/or obesity during childhood or adolescence can be associated with elevated blood pressure and an increased risk of renal disease progression, independent of birth weight
-
Micronutrient supplementation during pregnancy can reduce the risk of low birth weight and prematurity; the long-term impact on the risk of hypertension and kidney disease in these offspring remains unknown
Abstract
An adverse intrauterine environment is associated with an increased risk of elevated blood pressure and kidney disease in later life. Many studies have focused on low birth weight, prematurity and growth restriction as surrogate markers of an adverse intrauterine environment; however, high birth weight, exposure to maternal diabetes and rapid growth during early childhood are also emerging as developmental risk factors for chronic diseases. Altered programming of nephron number is an important link between exposure to developmental stressors and subsequent risk of hypertension and kidney disease. Maternal, fetal, and childhood nutrition are crucial contributors to these programming effects. Resource-poor countries experience the sequential burdens of fetal and childhood undernutrition and subsequent overnutrition, which synergistically act to augment the effects of developmental programming; this observation might explain in part the disproportionate burden of chronic disease in these regions. Numerous nutritional interventions have been effective in reducing the short-term risk of low birth weight and prematurity. Understanding the potential long-term benefits of such interventions is crucial to inform policy decisions to interrupt the developmental programming cycle and stem the growing epidemics of hypertension and kidney disease worldwide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systemayic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012).
Jha, V. et al. Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 (2013).
Couser, W. G., Remuzzi, G., Mendis, S. & Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 80, 1258–1270 (2011).
McMillen, I. C. & Robinson, J. S. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85, 571–633 (2005).
Barker, D. J. Developmental origins of adult health and disease. J. Epidemiol. Community Health 58, 114–115 (2004).
Barker, D. J. et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36, 62–67 (1993).
Whincup, P. H. et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 300, 2886–2897 (2008).
White, S. L. et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am. J. Kidney Dis. 54, 248–261 (2009).
Zetterstrom, K., Lindeberg, S., Haglund, B., Magnuson, A. & Hanson, U. Being born small for gestational age increases the risk of severe pre-eclampsia. BJOG 114, 319–324 (2007).
Nelson, R. G., Morgenstern, H. & Bennett, P. H. Intrauterine diabetes exposure and the risk of renal disease in diabetic Pima Indians. Diabetes 47, 1489–1493 (1998).
Zhang, Y. et al. The associations of high birth weight with blood pressure and hypertension in later life: a systematic review and meta-analysis. Hypertens. Res. 36, 725–735 (2013).
Hanson, M. & Gluckman, P. Developmental origins of noncommunicable disease: population and public health implications. Am. J. Clin. Nutr. 94, 1754S–1758S (2011).
Tzur, S. et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum. Genet. 128, 345–350 (2010).
Luyckx, V. A. et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 382, 273–283 (2013).
Uauy, R., Kain, J. & Corvalan, C. How can the Developmental Origins of Health and Disease (DOHaD) hypothesis contribute to improving health in developing countries? Am. J. Clin. Nutr. 94, 1759S–1764S (2011).
Yajnik, C. S. Transmission of obesity-adiposity and related disorders from the mother to the baby. Ann. Nutr. Metab. 64 (Suppl. 1), 8–17 (2014).
Adair, L. S. et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet 382, 525–534 (2013).
Black, R. E. et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382, 427–451 (2013).
Balbus, J. M. et al. Early-life prevention of non-communicable diseases. Lancet 381, 3–4 (2013).
Bhutta, Z. A. et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet 384, 347–370 (2014).
Bhutta, Z. A. et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 371, 417–440 (2008).
Lee, A. C. C. et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob. Health 1, e26–e36 (2013).
Mikolajczyk, R. T. et al. A global reference for fetal-weight and birthweight percentiles. Lancet 377, 1855–1861 (2011).
Schreuder, M. F., Langemeijer, M. E., Bokenkamp, A., Delemarre-Van de Waal, H. A. & Van Wijk, J. A. Hypertension and microalbuminuria in children with congenital solitary kidneys. J. Paediatr. Child. Health 44, 363–368 (2008).
Abou Jaoude, P. et al. Congenital versus acquired solitary kidney: is the difference relevant? Nephrol. Dial. Transplant. 26, 2188–2194 (2011).
Brenner, B. M., Garcia, D. L. & Anderson, S. Glomeruli and blood pressure. Less of one, more the other? Am. J. Hypertens. 1, 335–347 (1988).
Puelles, V. G. et al. Glomerular number and size variability and risk for kidney disease. Curr. Opin. Nephrol. Hypertens. 20, 7–15 (2011).
Sutherland, M. R. et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J. Am. Soc. Nephrol. 22, 1365–1374 (2011).
Lim, S. S. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions. 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260 (2012).
Mjoen, G. et al. Long-term risks for kidney donors. Kidney Int. 86, 162–167 (2014).
Muzaale, A. D. et al. Risk of end-stage renal disease following live kidney donation. JAMA 311, 579–586 (2014).
Berglund, D. et al. Low birthweight and risk of albuminuria in living kidney donors. Clin. Transplant. 28, 361–367 (2014).
Rogers, N. M., Lawton, P. D. & Jose, M. D. Indigenous Australians and living kidney donation. N. Engl. J. Med. 361, 1513–1516 (2009).
Gibney, E. M., Parikh, C. R. & Garg, A. X. Age, gender, race, and associations with kidney failure following living kidney donation. Transplant. Proc. 40, 1337–1340 (2008).
Storsley, L. J. et al. Long-term medical outcomes among Aboriginal living kidney donors. Transplantation 90, 401–406 (2010).
Nyengaard, J. R. Number and dimensions of rat glomerular capillaries in normal development and after nephrectomy. Kidney Int. 43, 1049–1057 (1993).
Singh, R. R. et al. Development of cardiovascular disease due to renal insufficiency in male sheep following fetal unilateral nephrectomy. J. Hypertens. 27, 386–396 (2009).
Baum, M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am. J. Physiol. Renal Physiol. 298, F235–F247 (2010).
Kett, M. M. & Denton, K. M. Renal programming: cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R791–R803 (2011).
Luyckx, V. A. & Brenner, B. M. The clinical importance of nephron mass. J. Am. Soc. Nephrol. 21, 898–910 (2010).
Stelloh, C. et al. Prematurity in mice leads to reduction in nephron number, hypertension, and proteinuria. Transl. Res. 159, 80–89 (2012).
Hughson, M., Farris, A. B., Douglas-Denton, R., Hoy, W. E. & Bertram, J. F. Glomerular number and size in autopsy kidneys: The relationship to birth weight. Kidney Int. 63, 2113–2122 (2003).
Zhang, Z. et al. A common RET variant is associated with reduced newborn kidney size and function. J. Am. Soc. Nephrol. 19, 2027–2034 (2008).
Nyengaard, J. R. & Bendtsen, T. F. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat. Rec. 232, 194–201 (1992).
Rakow, A. et al. Renal volume and function in school-age children born preterm or small for gestational age. Pediatr. Nephrol. 23, 1309–1315 (2008).
Kandasamy, Y., Smith, R., Wright, I. M. & Lumbers, E. R. Relationships between glomerular filtration rate and kidney volume in low-birth-weight neonates. J. Nephrol. 26, 894–898 (2013).
Bakker, H. et al. Fetal and infant growth patterns and kidney function at school age. J. Am. Soc. Nephrol. (2014).
Hoy, W. E. et al. The natural history of renal disease in Australian Aborigines. Part 2. Albuminuria predicts natural death and renal failure. Kidney Int. 60, 249–256 (2001).
Lemley, K. V. A basis for accelerated progression of diabetic nephropathy in Pima Indians. Kidney Int. Suppl. S38–S42 (2003).
Hoy, W. E., Hughson, M. D., Bertram, J. F., Douglas-Denton, R. & Amann, K. Nephron number, hypertension, renal disease, and renal failure. J. Am. Soc. Nephrol. 16, 2557–2564 (2005).
Rodriguez, M. M. et al. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr. Dev. Pathol. 7, 17–25 (2004).
Kalhan, S. C. & Wilson-Costello, D. Prematurity and programming: contribution of neonatal Intensive Care Unit interventions. J. Dev. Orig. Health Dis. 4, 121–133 (2013).
Hinchliffe, S. A., Lynch, M. R., Sargent, P. H., Howard, C. V. & Van Velzen, D. The effect of intrauterine growth retardation on the development of renal nephrons. Br. J. Obstet. Gynaecol. 99, 296–301 (1992).
de Jong, F., Monuteaux, M. C., van Elburg, R. M., Gillman, M. W. & Belfort, M. B. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 59, 226–234 (2012).
Mu, M. et al. Birth weight and subsequent blood pressure: a meta-analysis. Arch. Cardiovasc. Dis. 105, 99–113 (2012).
Keijzer-Veen, M. G., Dulger, A., Dekker, F. W., Nauta, J. & van der Heijden, B. J. Very preterm birth is a risk factor for increased systolic blood pressure at a young adult age. Pediatr. Nephrol. 25, 509–516 (2010).
Dalziel, S. R., Parag, V., Rodgers, A. & Harding, J. E. Cardiovascular risk factors at age 30 following pre-term birth. Int. J. Epidemiol. 36, 907–915 (2007).
Spence, D., Stewart, M. C., Alderdice, F. A., Patterson, C. C. & Halliday, H. L. Intra-uterine growth restriction and increased risk of hypertension in adult life: a follow-up study of 50-year-olds. Public Health 126, 561–565 (2012).
Bergvall, N. et al. Genetic and shared environmental factors do not confound the association between birth weight and hypertension: a study among Swedish twins. Circulation 115, 2931–2938 (2007).
Aceti, A. et al. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta-analysis. Diabetologia 55, 3114–3127 (2012).
Davis, E. F. et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics (2012).
Simonetti, G. D. et al. Salt sensitivity of children with low birth weight. Hypertension 52, 625–630 (2008).
de Boer, M. P. et al. Birth weight relates to salt sensitivity of blood pressure in healthy adults. Hypertension 51, 928–932 (2008).
Koyanagi, A. et al. Macrosomia in 23 developing countries: an analysis of a multicountry, facility-based, cross-sectional survey. Lancet 381, 476–483 (2013).
Keller, G., Zimmer, G., Mall, G., Ritz, E. & Amann, K. Nephron number in patients with primary hypertension. N. Engl. J. Med. 348, 101–108 (2003).
Hoy, W. E. et al. Nephron number, glomerular volume, renal disease and hypertension. Curr. Opin. Nephrol. Hypertens. 17, 258–265 (2008).
Hughson, M. D., Douglas-Denton, R., Bertram, J. F. & Hoy, W. E. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 69, 671–678 (2006).
Schreuder, M. F. et al. Impact of gestational age and birth weight on amikacin clearance on day 1 of life. Clin. J. Am. Soc. Nephrol. 4, 1774–1778 (2009).
Bacchetta, J. et al. Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int. 76, 445–452 (2009).
Keijzer-Veen, M. G. et al. Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J. Am. Soc. Nephrol. 16, 2762–2768 (2005).
Cassidy-Bushrow, A. E. et al. Race-specific relationship of birth weight and renal function among healthy young children. Pediatr. Nephrol. 27, 1317–1323 (2012).
Abi Khalil, C. et al. Fetal exposure to maternal type 1 diabetes is associated with renal dysfunction at adult age. Diabetes 59, 2631–2636 (2010).
Nelson, R. G., Morgenstern, H. & Bennett, P. H. Birth weight and renal disease in Pima Indians with type 2 diabetes mellitus. Am. J. Epidemiol. 148, 650–656 (1998).
Carmody, J. B. & Charlton, J. R. Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics 131, 1168–1179 (2013).
Rhone, E. T., Carmody, J. B., Swanson, J. R. & Charlton, J. R. Nephrotoxic medication exposure in very low birth weight infants. J. Matern. Fetal Neonatal Med. (2013).
Hsu, C. W., Yamamoto, K. T., Henry, R. K., De Roos, A. J. & Flynn, J. T. Prenatal risk factors for childhood CKD. J. Am. Soc. Nephrol. 25, 2105–2111 (2014).
Lackland, D. T., Bendall, H. E., Osmond, C., Egan, B. M. & Barker, D. J. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch. Intern. Med. 160, 1472–1476 (2000).
Li, S. et al. Low birth weight is associated with chronic kidney disease only in men. Kidney Int. 73, 637–642 (2008).
Vikse, B. E., Irgens, L. M., Leivestad, T., Hallan, S. & Iversen, B. M. Low birth weight increases risk for end-stage renal disease. J. Am. Soc. Nephrol. 19, 151–157 (2008).
Jackson, A. A., Dunn, R. L., Marchand, M. C. & Langley-Evans, S. C. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin. Sci. (Lond.) 103, 633–639 (2002).
Makrakis, J., Zimanyi, M. A. & Black, M. J. Retinoic acid enhances nephron endowment in rats exposed to maternal protein restriction. Pediatr. Nephrol. 22, 1861–1867 (2007).
Wlodek, M. E. et al. Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J. Am. Soc. Nephrol. 18, 1688–1696 (2007).
Godfrey, K. M. et al. Maternal nutritional status in pregnancy and blood pressure in childhood. Br. J. Obstet. Gynaecol. 101, 398–403 (1994).
Hult, M. et al. Hypertension, diabetes and overweight: looming legacies of the Biafran famine. PLoS ONE 5, e13582 (2010).
Painter, R. C. et al. Microalbuminuria in adults after prenatal exposure to the dutch famine. J. Am. Soc. Nephrol. 16, 189–194 (2005).
Stanner, S. A. et al. Does malnutrition in utero determine diabetes and coronary heart disease in adulthood? Results from the Leningrad siege study, a cross sectional study. BMJ 315, 1342–1348 (1997).
Stein, A. D., Zybert, P. A., van der Pal-de Bruin, K. & Lumey, L. H. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: evidence from the Dutch Famine. Eur. J. Epidemiol. 21, 759–765 (2006).
Bhutta, Z. A. et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 382, 452–477 (2013).
Lisle, S. J. et al. Effect of maternal iron restriction during pregnancy on renal morphology in the adult rat offspring. Br. J. Nutr. 90, 33–39 (2003).
Drake, K. A., Sauerbry, M. J., Blohowiak, S. E., Repyak, K. S. & Kling, P. J. Iron deficiency and renal development in the newborn rat. Pediatr. Res. 66, 619–624 (2009).
Tomat, A. L. et al. Moderate zinc restriction during fetal and postnatal growth of rats: effects on adult arterial blood pressure and kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R543–R549 (2008).
Altobelli, G., Bogdarina, I. G., Stupka, E., Clark, A. J. & Langley-Evans, S. Genome-Wide Methylation and Gene Expression Changes in Newborn Rats following Maternal Protein Restriction and Reversal by Folic Acid. PLoS ONE 8, e82989 (2013).
Vaidya, A. et al. Effects of antenatal multiple micronutrient supplementation on children's weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet 371, 492–499 (2008).
Brion, M. J., Leary, S. D., Smith, G. D., McArdle, H. J. & Ness, A. R. Maternal anemia, iron intake in pregnancy, and offspring blood pressure in the Avon Longitudinal Study of Parents and Children. Am. J. Clin. Nutr. 88, 1126–1133 (2008).
Hawkesworth, S. et al. Combined food and micronutrient supplements during pregnancy have limited impact on child blood pressure and kidney function in rural Bangladesh. J. Nutr. 143, 728–734 (2013).
Stewart, C. P. et al. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J. Nutr. 139, 1575–1581 (2009).
Merlet-Benichou, C., Vilar, J., Lelievre-Pegorier, M. & Gilbert, T. Role of retinoids in renal development: pathophysiological implication. Curr. Opin. Nephrol. Hypertens. 8, 39–43 (1999).
Goodyer, P. et al. Effects of maternal vitamin A status on kidney development: a pilot study. Pediatr. Nephrol. 22, 209–214 (2007).
El-Khashab, E. K., Hamdy, A. M., Maher, K. M., Fouad, M. A. & Abbas, G. Z. Effect of maternal vitamin A deficiency during pregnancy on neonatal kidney size. J. Perinat. Med. 41, 199–203 (2013).
Sutherland, M. R., Gubhaju, L., Yoder, B. A., Stahlman, M. T. & Black, M. J. The effects of postnatal retinoic acid administration on nephron endowment in the preterm baboon kidney. Pediatr. Res. 65, 397–402 (2009).
Thorne-Lyman, A. L. & Fawzi, W. W. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 26 (Suppl. 1), 36–54 (2012).
Christian, P. & Stewart, C. P. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J. Nutr. 140, 437–445 (2010).
Lee, L. M. et al. A paradoxical teratogenic mechanism for retinoic acid. Proc. Natl Acad. Sci. USA 109, 13668–13673 (2012).
Cresswell, J. A., Campbell, O. M., De Silva, M. J. & Filippi, V. Effect of maternal obesity on neonatal death in sub-Saharan Africa: multivariable analysis of 27 national datasets. Lancet 380, 1325–1330 (2012).
McDonald, S. D., Han, Z., Mulla, S., Beyene, J. & Knowledge Synthesis Group. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 341, c3428 (2010).
Yu, Z. et al. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS ONE 8, e61627 (2013).
Torloni, M. R. et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes. Rev. 10, 194–203 (2009).
Cnattingius, S., Villamor, E., Lagerros, Y. T., Wikstrom, A. K. & Granath, F. High birth weight and obesity—a vicious circle across generations. Int. J. Obes. (Lond.) (2011).
Filler, G. et al. Big mother or small baby: which predicts hypertension? J. Clin. Hypertens. (Greenwich) 13, 35–41 (2011).
Abalos, E. et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG 121 (Suppl. 1), 14–24 (2014).
Bilano, V. L., Ota, E., Ganchimeg, T., Mori, R. & Souza, J. P. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: a WHO secondary analysis. PLoS ONE 9, e91198 (2014).
Boivin, A. et al. Pregnancy complications among women born preterm. CMAJ 184, 1777–1784 (2012).
Manalich, R., Reyes, L., Herrera, M., Melendi, C. & Fundora, I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 58, 770–773 (2000).
Kanguru, L., Bezawada, N., Hussein, J. & Bell, J. The burden of diabetes mellitus during pregnancy in low- and middle-income countries: a systematic review. Glob. Health Action 7, 23987 (2014).
Amri, K., Freund, N., Vilar, J., Merlet-Benichou, C. & Lelievre-Pegorier, M. Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes 48, 2240–2245 (1999).
Tran, S. et al. Maternal diabetes modulates renal morphogenesis in offspring. J. Am. Soc. Nephrol. 19, 943–952 (2008).
Seghieri, G. et al. Relationship between gestational diabetes mellitus and low maternal birth weight. Diabetes Care 25, 1761–1765 (2002).
Vidal, A. C. et al. Associations between antibiotic exposure during pregnancy, birth weight and aberrant methylation at imprinted genes among offspring. Int. J. Obes. (Lond.) 37, 907–913 (2013).
Gilbert, T., Lelievre-Pegorier, M. & Merlet-Benichou, C. Immediate and long-term renal effects of fetal exposure to gentamicin. Pediatr. Nephrol. 4, 445–450 (1990).
Nathanson, S., Moreau, E., Merlet-Benichou, C. & Gilbert, T. In utero and in vitro exposure to beta-lactams impair kidney development in the rat. J. Am. Soc. Nephrol. 11, 874–884 (2000).
Merlet-Benichou, C. Influence of fetal environment on kidney development. Int. J. Dev. Biol. 43, 453–456 (1999).
Tendron-Franzin, A. et al. Long-term effects of in utero exposure to cyclosporin A on renal function in the rabbit. J. Am. Soc. Nephrol. 15, 2687–2693 (2004).
McKay, D. B. & Josephson, M. A. Pregnancy in recipients of solid organs—effects on mother and child. N. Engl. J. Med. 354, 1281–1293 (2006).
Komhoff, M. et al. Cyclooxygenase-2-selective inhibitors impair glomerulogenesis and renal cortical development. Kidney Int. 57, 414–422 (2000).
Kent, A. L. et al. Indomethacin, ibuprofen and gentamicin administered during late stages of glomerulogenesis do not reduce glomerular number at 14 days of age in the neonatal rat. Pediatr. Nephrol. 24, 1143–1149 (2009).
Langley-Evans, S. C. et al. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta 17, 169–172 (1996).
Dalziel, S. R. et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet 365, 1856–1862 (2005).
Nykjaer, C. et al. Maternal alcohol intake prior to and during pregnancy and risk of adverse birth outcomes: evidence from a British cohort. J. Epidemiol. Community Health 68, 542–549 (2014).
Gray, S. P. et al. Repeated ethanol exposure during late gestation decreases nephron endowment in fetal sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R568–R574 (2008).
Gray, S. P., Denton, K. M., Cullen-McEwen, L., Bertram, J. F. & Moritz, K. M. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J. Am. Soc. Nephrol. 21, 1891–1902 (2010).
Taal, H. R. et al. Maternal smoking during pregnancy and kidney volume in the offspring: the Generation R Study. Pediatr. Nephrol. 26, 1275–1283 (2011).
Jagadapillai, R. et al. Developmental cigarette smoke exposure: kidney proteome profile alterations in low birth weight pups. Toxicology 299, 80–89 (2012).
Chevalier, R. L. Chronic partial ureteral obstruction and the developing kidney. Pediatr. Radiol. 38 (Suppl. 1), S35–S40 (2008).
Chevalier, R. L., Thornhill, B. A., Chang, A. Y., Cachat, F. & Lackey, A. Recovery from release of ureteral obstruction in the rat: relationship to nephrogenesis. Kidney Int. 61, 2033–2043 (2002).
Chevalier, R. L., Thornhill, B. A. & Chang, A. Y. Unilateral ureteral obstruction in neonatal rats leads to renal insufficiency in adulthood. Kidney Int. 58, 1987–1995 (2000).
Ismaili, K. et al. Results of systematic screening for minor degrees of fetal renal pelvis dilatation in an unselected population. Am. J. Obstet. Gynecol. 188, 242–246 (2003).
Ibrahim, A. G., Aliyu, S. & Ali, N. Bilateral pelvi-ureteric junction obstruction: our experience in a developing country. Niger. J. Clin. Pract. 17, 267–269 (2014).
Ocheke, I. E., Antwi, S., Gajjar, P., McCulloch, M. I. & Nourse, P. Pelvi-ureteric junction obstruction at Red Cross Children's Hospital, Cape Town: a six year review. Arab J. Nephrol. Transplant. 7, 33–36 (2014).
Christian, P. et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int. J. Epidemiol. 42, 1340–1355 (2013).
Rossing, P. et al. Short stature and diabetic nephropathy. BMJ 310, 296–297 (1995).
Sichieri, R., Siqueira, K. S., Pereira, R. A. & Ascherio, A. Short stature and hypertension in the city of Rio de Janeiro, Brazil. Public Health Nutr. 3, 77–82 (2000).
Tennant, I. A. et al. Impaired cardiovascular structure and function in adult survivors of severe acute malnutrition. Hypertension 64, 664–671 (2014).
Zhao, Y., Wang, S. F., Mu, M. & Sheng, J. Birth weight and overweight/obesity in adults: a meta-analysis. Eur. J. Pediatr. (2012).
Skelton, J. A., Irby, M. B., Grzywacz, J. G. & Miller, G. Etiologies of obesity in children: nature and nurture. Pediatr. Clin. North Am. 58, 1333–1354, ix (2011).
Richardson, L. J., Hussey, J. M. & Strutz, K. L. Origins of disparities in cardiovascular disease: birth weight, body mass index, and young adult systolic blood pressure in the national longitudinal study of adolescent health. Ann. Epidemiol. 21, 598–607 (2011).
Law, C. M. et al. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation 105, 1088–1092 (2002).
Hemachandra, A. H., Howards, P. P., Furth, S. L. & Klebanoff, M. A. Birth weight, postnatal growth, and risk for high blood pressure at 7 years of age: results from the Collaborative Perinatal Project. Pediatrics 119, e1264–e1270 (2007).
Jain, V. & Singhal, A. Catch up growth in low birth weight infants: striking a healthy balance. Rev. Endocr. Metab. Disord. 13, 141–147 (2012).
Andersen, L. G. et al. Birth weight, childhood body mass index and risk of coronary heart disease in adults: combined historical cohort studies. PLoS ONE 5, e14126 (2010).
Barker, D. J., Osmond, C., Forsen, T. J., Kajantie, E. & Eriksson, J. G. Trajectories of growth among children who have coronary events as adults. N. Engl. J. Med. 353, 1802–1809 (2005).
Fall, C. H. et al. Adult metabolic syndrome and impaired glucose tolerance are associated with different patterns of BMI gain during infancy: Data from the New Delhi Birth Cohort. Diabetes Care 31, 2349–2356 (2008).
Silverwood, R. J. et al. Low birth weight, later renal function, and the roles of adulthood blood pressure, diabetes, and obesity in a British birth cohort. Kidney Int. 84, 1262–1270 (2013).
Kwinta, P. et al. Assessment of long-term renal complications in extremely low birth weight children. Pediatr. Nephrol. 26, 1095–1103 (2011).
Boubred, F. et al. Effects of early postnatal hypernutrition on nephron number and long-term renal function and structure in rats. Am. J. Physiol. Renal Physiol. 293, F1944–F1949 (2007).
Abitbol, C. L. et al. Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatr. Nephrol. 18, 887–893 (2003).
Abitbol, C. L. et al. Obesity and preterm birth: additive risks in the progression of kidney disease in children. Pediatr. Nephrol. 24, 1363–1370 (2009).
Yim, H. E. & Yoo, K. H. Early life obesity and chronic kidney disease in later life. Pediatr. Nephrol. (2014).
Moritz, K. M. et al. Review: Sex specific programming: a critical role for the renal renin-angiotensin system. Placenta 31 (Suppl.), S40–S46 (2010).
Laaksonen, D. E. et al. Cardiorespiratory fitness and vigorous leisure-time physical activity modify the association of small size at birth with the metabolic syndrome. Diabetes Care 26, 2156–2164 (2003).
Huxley, R. R., Shiell, A. W. & Law, C. M. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J. Hypertens. 18, 815–831 (2000).
Webb, A. L. et al. Maternal and childhood nutrition and later blood pressure levels in young Guatemalan adults. Int. J. Epidemiol. 34, 898–904 (2005).
Kinra, S. et al. Effect of integration of supplemental nutrition with public health programmes in pregnancy and early childhood on cardiovascular risk in rural Indian adolescents: long term follow-up of Hyderabad nutrition trial. BMJ 337, a605 (2008).
Hawkesworth, S., Prentice, A. M., Fulford, A. J. & Moore, S. E. Maternal protein-energy supplementation does not affect adolescent blood pressure in The Gambia. Int. J. Epidemiol. 38, 119–127 (2009).
Stewart, C. P. et al. Maternal supplementation with vitamin A or beta-carotene and cardiovascular risk factors among pre-adolescent children in rural Nepal. J. Dev. Origins Health Dis. 1, 262–270 (2010).
Bergel, E. & Barros, A. J. Effect of maternal calcium intake during pregnancy on children's blood pressure: a systematic review of the literature. BMC Pediatr. 7, 15 (2007).
Hawkesworth, S. et al. Effect of maternal calcium supplementation on offspring blood pressure in 5- to 10-y-old rural Gambian children. Am. J. Clin. Nutr. 92, 741–747 (2010).
Abitbol, C. L. & Rodriguez, M. M. The long-term renal and cardiovascular consequences of prematurity. Nat. Rev. Nephrol. 8, 265–274 (2012).
Abou-Jaoude, P. et al. What about the renal function during childhood of children born from dialysed mothers? Nephrol. Dial. Transplant. 27, 2365–2369 (2012).
Blumenshine, P., Egerter, S., Barclay, C. J., Cubbin, C. & Braveman, P. A. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am. J. Prev. Med. 39, 263–272 (2010).
Ganchimeg, T. et al. Pregnancy and childbirth outcomes among adolescent mothers: a World Health Organization multicountry study. BJOG 121 (Suppl. 1), 40–48 (2014).
Gavin, A. R., Thompson, E., Rue, T. & Guo, Y. Maternal early life risk factors for offspring birth weight: findings from the add health study. Prev. Sci. 13, 162–172 (2012).
Kinabo, J. Seasonal variation of birth weight distribution in Morogoro, Tanzania. East Afr. Med. J. 70, 752–755 (1993).
Lawoyin, T. O. & Oyediran, A. B. A prospective study on some factors which influence the delivery of low birth weight babies in a developing country. Afr. J. Med. Med. Sci. 21, 33–39 (1992).
Mansour, H. & Rees, D. I. Armed conflict and birth weight: Evidence from the al-Aqsa Intifada. J. Dev. Economics 99, 190–199 (2012).
Morken, N. H., Travlos, G. S., Wilson, R. E., Eggesbo, M. & Longnecker, M. P. Maternal glomerular filtration rate in pregnancy and fetal size. PLoS ONE 9, e101897 (2014).
Ota, E. et al. Risk factors and adverse perinatal outcomes among term and preterm infants born small-for-gestational-age: secondary analyses of the WHO multi-country survey on maternal and newborn health. PLoS ONE 9, e105155 (2014).
Rao, S., Kanade, A. N., Yajnik, C. S. & Fall, C. H. Seasonality in maternal intake and activity influence offspring's birth size among rural Indian mothers—Pune Maternal Nutrition Study. Int. J. Epidemiol. 38, 1094–1103 (2009).
Walker, P. G., Ter Kuile, F. O., Garske, T., Menendez, C. & Ghani, A. C. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob. Health 2, e460–e467 (2014).
WHO. Meeting of Advisory Group on Maternal Nutrition and Low Birth Weight, Geneva. World Health Organization (2002).
Author information
Authors and Affiliations
Contributions
V.A.L. researched the data for the article, contributed substantially to the discussion of content and wrote the article. V.A.L. and B.M.B. contributed equally to the review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Global prevalence of micronutrient deficiencies associated with low birth weight. (PDF 329 kb)
Supplementary Figure 2
Incidence of gestational hypertension by WHO geographical region. (PDF 307 kb)
Supplementary Table 1
Clinical associations with nephron number and kidney size. (PDF 72 kb)
Rights and permissions
About this article
Cite this article
Luyckx, V., Brenner, B. Birth weight, malnutrition and kidney-associated outcomes—a global concern. Nat Rev Nephrol 11, 135–149 (2015). https://doi.org/10.1038/nrneph.2014.251
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2014.251
This article is cited by
-
Sirtuins in kidney health and disease
Nature Reviews Nephrology (2024)
-
Early post-natal nutrition and renal consequences in preterm infants
Pediatric Research (2024)
-
Kidney growth following preterm birth: evaluation with renal parenchyma ultrasonography
Pediatric Research (2023)
-
Inequities in kidney health and kidney care
Nature Reviews Nephrology (2023)
-
Prävention ungünstiger metabolischer Prägung bei intrauteriner Wachstumsrestriktion
Die Diabetologie (2023)