Abstract
The stimulation of erythropoiesis is a rapidly evolving area of research, with mechanistic insights often developing rapidly into therapeutic agents. A broad range of erythropoiesis-stimulating agents are currently in clinical use and many more under development are likely to enter the marketplace in the near future. To date, much of the investigative activity in this field has targeted activation of the erythropoietin receptor and factors that modulate hypoxia-related pathways of erythropoietin production within cells. This Review discusses newer erythropoiesis-stimulating agents currently under assessment for the treatment of anemia in patients with chronic kidney disease. Such agents include proteins and peptides that activate erythropoietin receptors, non-protein agents, and strategies with targets other than erythropoietin receptors.
Key Points
-
Considerable advances have been made in our understanding of the mechanisms that link oxygen sensing and erythropoiesis
-
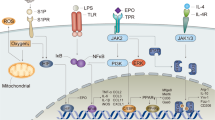
Knowledge of the biology of erythropoietin production and the function of the erythropoietin receptor is critical for understanding and developing new therapeutic agents for anemia
-
Most novel erythropoiesis-stimulating agents (ESAs) involve stimulation of the erythropoietin receptor; post-translational modifications of the erythropoietin protein can affect the duration of action of ESAs
-
Chemically synthesized peptides that act on the erythropoietin receptor are also in clinical development
-
Non-peptide agents that directly or indirectly stimulate the erythropoietin gene (EPO) have the potential to become oral ESAs in the future
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Fisher, J. W. Erythropoietin: physiologic and pharmacologic aspects. Proc. Soc. Exp. Biol. Med. 16, 358–369 (1997).
Adamson, J. W. Erythropoietin: in vitro and in vivo studies of the regulation of erythropoiesis. Schweiz Med. Wochenschr. 118, 1501–1506 (1988).
Jelkmann, W. Erythropoietin: structure, control of production, and function. Physiol. Rev. 72, 449–489 (1992).
Salmonson, T., Danielson, B. G. & Wikström, B. The pharmacokinetics of recombinant human erythropoietin after intravenous and subcutaneous administration to healthy subjects. Br. J. Clin. Pharmacol. 29, 709–713 (1990).
Jelkmann, W. Control of erythropoietin gene expression and its use in medicine. Methods Enzymol. 435, 179–197 (2007).
Wang, G. L. & Semenza, G. L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl Acad. Sci. USA 90, 4304–4308 (1993).
Semenza, G. L. HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107, 1–3 (2001).
Zhu, H. & Bunn, H. F. Signal transduction: how do cells sense oxygen? Science 292, 449–451 (2001).
Prchal, J. T. Delivery on demand a new era of gene therapy? N. Engl. J. Med. 348, 1282–1283 (2003).
Bruegge, K., Jelkmann, W. & Metzen, E. Hydroxylation of hypoxia-inducible transcription factors and chemical compounds targeting the HIF-alpha hydroxylases. Curr. Med. Chem. 14, 1853–1862 (2007).
Nakano, Y. et al. Oral administration of K-11706 inhibits GATA binding activity, enhances hypoxia-inducible factor 1 binding activity, and restores indicators in an in vivo mouse model of anemia of chronic disease. Blood 104, 4300–4307 (2004).
Sasaki, H., Bothner, B., Dell, A. & Fukuda, M. Carbohydrate structure of erythropoietin expressed in Chinese hamster ovary cells by a human erythropoietin cDNA. J. Biol. Chem. 262, 12059–12076 (1987).
Takeuchi, M. & Kobata, A. Structures and functional roles of the sugar chains of human erythropoietins. Glycobiology 4, 337–346 (1991).
Rush, R. S. et al. Microheterogeneity of erythropoietin carbohydrate structure. Anal. Chem. 67, 1442–1452 (1995).
Egrie, J. C. & Browne, J. K. Development and characterization of darbepoetin alfa. Oncology (Williston Park) 16 (Suppl. 11), 13–22 (2002).
Higuchi, M. et al. Role of sugar chains in the expression of the biological activity of human erythropoietin. J. Biol. Chem. 267, 7703–7709 (1992).
Digicaylioglu, M. et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc. Natl Acad. Sci. USA 92, 3717–3720 (1995).
Juul, S. E., Yachnis, A. T. & Christensen, R. D. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum. Dev. 52, 235–249 (1998).
Anagnostou, A. et al. Erythropoietin receptor mRNA expression in human endothelial cells. Proc. Natl Acad. Sci. USA 91, 3974–3978 (1994).
Rossert, J. & Eckardt, K. U. Erythropoietin receptors: their role beyond erythropoiesis. Nephrol. Dial. Transplant. 20, 1025–1028 (2005).
Lacombe, C. & Mayeux, P. The molecular biology of erythropoietin. Nephrol. Dial. Transplant. 14, (Suppl. 2), 22–28 (1999).
Ratajczak, J. et al. Biological significance of MAPK, AKT and JAK-STAT protein activation by various erythropoietic factors in normal human early erythroid cells. Br. J. Haematol. 115, 195–204 (2001).
Silva, M. et al. Erythropoietin can induce the expression of bcl-x(L) through Stat5 in erythropoietin-dependent progenitor cell lines. J. Biol. Chem. 274, 22165–22169 (1999).
Kashii, Y. et al. A member of Forkhead family transcription factor, FKHRL1, is one of the downstream molecules of phosphatidylinositol 3-kinase-Akt activation pathway in erythropoietin signal transduction. Blood 96, 941–949 (2000).
Yang, C. W. et al. Preconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in rat kidney. FASEB J. 17, 1754–1755 (2003).
Locatelli, F. et al. Novel erythropoiesis stimulating protein for treatment of anemia in chronic renal insufficiency. Kidney Int. 60, 741–747 (2001).
Nissenson, A. R. et al. Randomized, controlled trial of darbepoetin alfa for the treatment of anemia in hemodialysis patients. Am. J. Kidney Dis. 40, 110–118 (2002).
Vanrenterhem, Y. et al. Randomized trial of darbepoetin alfa for treatment of renal anemia at a reduced dose frequency compared with rHuEPO in dialysis patients. Kidney Int. 62, 2167–2175 (2002).
Warady, B. A. et al. Darbepoetin alfa for the treatment of anemia in pediatric patients with chronic kidney disease. Pediatr. Nephrol. 21, 1144–1152 (2006).
Levin, N. W. et al. Intravenous methoxy polyethylene glycol-epoetin beta for haemoglobin control in patients with chronic kidney disease who are on dialysis: a randomized non-inferiority trial (MAXIMA). Lancet 370, 1415–1421 (2007).
Klinger, M. et al. Efficacy of intravenous methoxy polyethylene glycol-epoetin beta administered every 2 weeks compared with epoetin administered 3 times weekly in patients treated by hemodialysis or peritoneal dialysis: a randomized trial. Am. J. Kidney. Dis. 50, 989–1000 (2007).
Sulowicz, W. et al. Once-monthly subcutaneous C. E. R. A. maintains stable hemoglobin control in patients with chronic kidney disease on dialysis and converted directly from epoetin one to three times weekly. Clin. J. Am. Soc. Nephrol. 2, 637–646 (2007).
Canaud, B. et al. Intravenous C. E. R. A. maintains stable haemoglobin levels in patients on dialysis previously treated with darbepoetin alfa: results from STRIATA, a randomized phase III study. Nephrol. Dial. Transplant. 23, 3654–3661 (2008).
Macdougall, I. C. et al. C. E. R. A. corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trial. Clin. J. Am. Soc. Nephrol. 3, 337–347 (2008).
Spinowitz, B. et al. C. E. R. A. maintains stable control of hemoglobin in patients with chronic kidney disease on dialysis when administered once every two weeks. Am. J. Nephrol. 28, 280–289 (2008).
European Public Assessment Report (EPAR) and Summary of Product Characteristics Epoetin Alfa Hexal [online], (2009).
Schellekens, H. The first biosimilar epoetin: but how similar is it? Clin. J. Am. Soc. Nephrol. 3, 174–178 (2008).
Jounga, J., Robertson, J. S, Griffiths, E. & Knezevica, I. ; on behalf of the WHO Informal Consultation Group. Meeting Report: WHO Informal Consultation on Regulatory Evaluation of Therapeutic Biological Medicinal Products [online], (2009).
Haag-Weber. M., Vetter, A., Thyroff-Friesinger, U. & INJ-Study Group. Therapeutic equivalence, long-term efficacy and safety of HX575 in the treatment of anemia in chronic renal failure patients receiving hemodialysis. Clin. Nephrol. 72, 380–390 (2009).
Casadevall, N. et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N. Engl. J. Med. 346, 469–475 (2002).
Peces, R., de la Torre, M., Alcazar, R. & Urra, J. M. Antibodies against recombinant human erythropoietin in a patient with erythropoietin-resistant anemia. N. Engl. J. Med. 335, 523–524 (1996).
Gershon, S. K., Luksenburg, H., Coté, T. R. & Braun, M. M. Pure red-cell aplasia and recombinant erythropoietin. N. Engl. J. Med. 346, 1584–1585 (2002).
Bennett, C. L. et al. Pure red-cell aplasia and epoetin therapy. N. Engl. J. Med. 351, 1403–1408 (2004).
Deicher, R. & Hörl, W. H. Differentiating factors between erythropoiesis-stimulating agents: a guide to selection for anaemia of chronic kidney disease. Drugs 64, 499–509 (2004).
Llop, E. et al. Structural analysis of the glycosylation of gene-activated erythropoietin (epoetin delta, Dynepo). Anal. Biochem. 383, 243–254 (2008).
Spinowitz, B. S. & Pratt, R. D. Epoetin delta is effective for the management of anaemia associated with chronic kidney disease. Curr. Med. Res. Opin. 22, 2507–2513 (2006).
Martin, K. J. The first human cell line-derived erythropoietin, epoetin-delta (Dynepo), in the management of anemia in patients with chronic kidney disease. Clin. Nephrol. 68, 26–31 (2007).
Martin, K. J. & Epoetin Delta 3001 Study Group. Epoetin delta in the management of renal anaemia: results of a 6-month study. Nephrol. Dial. Transplant. 22, 3052–3054 (2007).
Kwan, J. T., Pratt, R. D. & Epoetin Delta Study Group. Epoetin delta, erythropoietin produced in a human cell line, in the management of anaemia in predialysis chronic kidney disease patients. Curr. Med. Res. Opin. 23, 307–311 (2007).
Spinowitz, B. S., Pratt, R. D. & Epoetin Delta 2002 Study Group. Epoetin delta is effective for the management of anaemia associated with chronic kidney disease. Curr. Med. Res. Opin. 22, 2507–2513 (2006).
European Medicines Agency. Public Statement on Dynepo (Epoetin Delta) [online], (2009).
Sikole, A., Spasovski, G., Zafirov, D. & Polenakovic, M. Epoetin omega for treatment of anemia in maintenance hemodialysis patients. Clin. Nephrol. 57, 237–245 (2002).
Acharya, V. N., Sinha, D. K., Almeida, A. F. & Pathare, A. V. Effect of low dose recombinant human omega erythropoietin (rHuEPO) on anaemia in patients on hemodialysis. J. Assoc. Physicians India 43, 539–542 (1995).
Bren, A. et al. A comparison between epoetin omega and epoetin alfa in the correction of anemia in hemodialysis patients: a prospective, controlled crossover study. Artif. Organs 26, 91–97 (2002).
Milutinović, S., Plavljanić, E. & Trkulja, V. Comparison of two epoetin brands in anemic hemodialysis patients: results of two efficacy trials and a single-dose pharmacokinetic study. Fundam. Clin. Pharmacol. 20, 493–502 (2006).
Egrie, J. C. & Browne, J. K. Development and characterization of novel erythropoiesis stimulating protein (NESP). Br. J. Cancer 84 (Suppl. 1), 3–10 (2001).
Macdougall, I. C. et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J. Am. Soc. Nephrol. 10, 2392–2395 (1999).
Padhi, D. et al. An extended terminal half-life for darbepoetin alfa: results from a single-dose pharmacokinetic study in patients with chronic kidney disease not receiving dialysis. Clin. Pharmacokinet. 45, 503–510 (2006).
McKoy, J. M. et al. Epoetin-associated pure red cell aplasia: past, present, and future considerations. Transfusion 48, 1754–1762 (2008).
Roger, S. D. et al. A randomised, cross-over study comparing injection site pain with subcutaneous epoetin beta and subcutaneous darbepoetin alfa in patients with chronic kidney disease. Curr. Med. Res. Opin. 24, 2181–2187 (2008).
Macdougall, I. C. CERA (Continuous Erythropoietin Receptor Activator): a new erythropoiesis-stimulating agent for the treatment of anemia. Curr. Hematol. Rep. 6, 436–440 (2005).
Macdougall, I. C. et al. Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C. E. R. A.) in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 1, 1211–1215 (2006).
McKoy, J. M. et al. Epoetin-associated pure red cell aplasia: past, present, and future considerations. Transfusion 48, 1754–1762 (2008).
Bouman-Thio, E. et al. A phase I, single and fractionated, ascending-dose study evaluating the safety, pharmacokinetics, pharmacodynamics, and immunogenicity of an erythropoietin mimetic antibody fusion protein (CNTO 528) in healthy male subjects. J. Clin. Pharmacol. 48, 1197–1207 (2008).
Wrighton, N. C. et al. Small peptides as potent mimetics of the protein hormone erythropoietin. Science 273, 458–464 (1996).
Stead, R. B. Evaluation of the safety and pharmacodynamics of Hematide, a novel erythropoietic agent, in a phase 1, double-blind, placebo-controlled, dose-escalation study in healthy volunteers. Blood 108, 1830–1834 (2006).
Woodburn, K. W. et al. Hematide is immunologically distinct from erythropoietin and corrects anemia induced by antierythropoietin antibodies in a rat pure red cell aplasia model. Exp. Hematol. 35, 1201–1208 (2007).
Macdougall, I. C. et al. A peptide-based erythropoietin-receptor agonist for pure red-cell aplasia. N. Engl. J. Med. 361, 1848–1855 (2009).
Macdougall, I. C. Novel erythropoiesis-stimulating agents: a new era in anemia management. Clin. J. Am. Soc. Nephrol. 3, 200–207 (2008).
Binley, K. et al. Long-term reversal of chronic anemia using a hypoxia-regulated erythropoietin gene therapy. Blood 100, 2406–2413 (2002).
Clinical Trials. Safety and Efficacy of Sustained Erythropoietin Therapy [online], (2009).
Unger, E. F., Thompson, A. M., Blank, M. J. & Temple, R. Erythropoiesis-stimulating agents—time for a reevaluation. N. Engl. J. Med. 362, 189–192 (2010).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares associations with the following companies: Affymax, Amgen, Ortho Biotech and Roche (consulting fees).
Rights and permissions
About this article
Cite this article
Foley, R. Emerging erythropoiesis-stimulating agents. Nat Rev Nephrol 6, 218–223 (2010). https://doi.org/10.1038/nrneph.2010.19
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.19
This article is cited by
-
Epoetin beta pegol (C.E.R.A.) promotes utilization of iron for erythropoiesis through intensive suppression of serum hepcidin levels in mice
International Journal of Hematology (2014)
-
Erythropoietin stimulation decreases hepcidin expression through hematopoietic activity on bone marrow cells in mice
International Journal of Hematology (2012)