Key Points
-
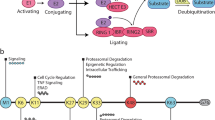
The 76-amino-acid protein ubiquitin was initially identified as a tag that marks proteins for degradation by the proteasome. However, ubiquitination can also serve as a rapidly reversible mechanism for the covalent modification of proteins, and recent studies have highlighted its importance for the rapid modification of proteins in the brain.
-
The substrates and enzymes of the ubiquitin pathway can be regulated by phosphorylation, and a new mode of regulation by ubiquitin-like proteins has also been discovered.
-
Ubiquitin-regulated protein degradation provides a mechanism that links axon-guidance cues — such as the netrins, semaphorins, ephrins and Slit — to growth cone dynamics. From work on the Drosophila neuromuscular junction, ubiquitination has also been implicated as a key regulator of synapse formation and growth.
-
Synaptic strength can be regulated by controlling the abundance of neurotransmitter receptors at the synapse, through targeted insertion and removal. Ubiquitination is a signal for endocytosis, so it is an attractive candidate mechanism for controlling the levels of neurotransmitter receptors. Support for this hypothesis has come from work with the inhibitory glycine and GABAA (γ-aminobutyric acid type A) receptors, and the excitatory glutamate receptors.
-
The ubiquitin pathway also seems to be involved in synaptic plasticity. In Aplysia, it has been shown that ubiquitination of cyclic-AMP-dependent protein kinase causes it to remain active, even at low levels of cAMP, and this persistent activity helps to convert short-term synaptic facilitation to a longer-lasting phenomenon.
-
So, several functions of ubiquitin in the nervous system have already been discovered, and it is expected that ubiquitination will join phosphorylation as a central mechanism for modifying neuronal activity.
Abstract
Post-translational modification by the attachment of ubiquitin seems to have a crucial role in regulating synaptic structure and function. By controlling the stability, activity and localization of target proteins, this versatile regulatory system can shape the pattern, activity and plasticity of synaptic connections. Here, we discuss the myriad ways in which ubiquitin functions to sculpt synapses during development, and to remodel synapses for the acquisition and storage of memory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ciechanover, A., Heller, H., Elias, S., Haas, A. L. & Hershko, A. ATP-dependent conjugation of reticulocyte proteins with the polypeptide required for protein degradation. Proc. Natl Acad. Sci. USA 77, 1365–1368 (1980).
Hershko, A., Ciechanover, A., Heller, H., Haas, A. L. & Rose, I. A. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc. Natl Acad. Sci. USA 77, 1783–1786 (1980).
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998).
Glickman, M. H. & Ciechanover, A. The ubiquitin–proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82, 373–428 (2002).
Tanaka, K. & Chiba, T. The proteasome: a protein-destroying machine. Genes Cells 3, 499–510 (1998).
Patrick, G. N., Zhou, P., Kwon, Y. T., Howley, P. M. & Tsai, L. H. p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin–proteasome pathway. J. Biol. Chem. 273, 24057–24064 (1998).
Yamamoto, Y., Hegde, A. N., Chain, D. G. & Schwartz, J. H. Activation and degradation of the transcription factor C/EBP during long-term facilitation in Aplysia. J. Neurochem. 73, 2415–2423 (1999).This paper shows that a transcription factor required for the induction of long-term synaptic plasticity (Ap-C/EBP) is degraded by the ubiquitin–proteasome pathway, and that phosphorylation by MAP kinase makes the transcription factor resistant to ubiquitin-mediated degradation.
Musti, A. M., Treier, M. & Bohmann, D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science 275, 400–402 (1997).
Schwarzschild, M. A., Cole, R. L. & Hyman, S. E. Glutamate, but not dopamine, stimulates stress-activated protein kinase and AP-1-mediated transcription in striatal neurons. J. Neurosci. 17, 3455–3466 (1997).
Pulverer, B. J., Kyriakis, J. M., Avruch, J., Nikolakaki, E. & Woodgett, J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature 353, 670–674 (1991).
Gieffers, C., Peters, B. H., Kramer, E. R., Dotti, C. G. & Peters, J. M. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc. Natl Acad. Sci. USA 96, 11317–11322 (1999).
Lahav-Baratz, S., Sudakin, V., Ruderman, J. V. & Hershko, A. Reversible phosphorylation controls the activity of cyclosome associated cyclin-ubiquitin ligase. Proc. Natl Acad. Sci. USA 92, 9303–9307 (1995).
Kauselmann, G. et al. The polo-like protein kinases Fnk and Snk associate with a Ca2+- and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO J. 20, 5528–5539 (1999).
Hochstrasser, M. Evolution and function of ubiquitin-like protein-conjugation systems. Nature Cell Biol. 2, E153–E157 (2000).
Wilkinson, K. D. Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin. Cell Dev. Biol. 11, 141–148 (2000).
Hadari, T., Warms, J. V., Rose, I. A. & Hershko, A. A ubiquitin C-terminal isopeptidase that acts on polyubiquitin chains. Role in protein degradation. J. Biol. Chem. 267, 719–727 (1992).
DiAntonio, A. et al. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature 412, 449–452 (2001).This work investigates how the balance between factors that promote and antagonize ubiquitination can regulate the growth and function of synapses at the Drosophila neuromuscular junction.
Hegde, A. N. et al. Ubiquitin C-terminal hydrolase is an immediate–early gene essential for long-term facilitation in Aplysia. Cell 89, 115–126 (1997).Using molecular-biological, biochemical and electrophysiological techniques, this paper shows that an enzyme of the ubiquitin–proteasome pathway (Ap-uch) is crucial for the formation of a simple form of long-term memory.
Muralidhar, M. G. & Thomas, J. B. The Drosophila bendless gene encodes a neural protein related to ubiquitin-conjugating enzymes. Neuron 11, 253–266 (1993).
Oh, C. et al. bendless, a Drosophila gene affecting neuronal connectivity, encodes a ubiquitin-conjugating enzyme homolog. J. Neurosci. 14, 3166–3179 (1994).
Hegde, A. N., Goldberg, A. L. & Schwartz, J. H. Regulatory subunits of the cAMP-dependent protein kinases are degraded after conjugation to ubiquitin: a molecular mechanism underlying long-term synaptic plasticity. Proc. Natl Acad. Sci. USA 90, 7436–7440 (1993).The first demonstration that a subunit of a protein kinase (PKA) that is crucial for synaptic plasticity is a substrate of the ubiquitin–proteasome pathway.
Yu, T. W. & Bargmann, C. I. Dynamic regulation of axon guidance. Nature Neurosci. 4 (Suppl.), 1169–1176 (2001).
Zhang, L. I. & Poo, M. M. Electrical activity and development of neural circuits. Nature Neurosci. 4 (Suppl.), 1207–1214 (2001).
Doherty, P. G. et al. CAMs and axonal growth: a critical evaluation of the role of calcium and the MAPK cascade. Mol. Cell. Neurosci. 16, 283–295 (2000).
Korey, C. A. & Van Vactor, D. From the growth cone surface to the cytoskeleton: one journey, many paths. J. Neurobiol. 44, 184–193 (2000).
Campbell, D. S. & Holt, C. E. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32, 1013–1026 (2001).This study shows that axon-guidance cues can induce ubiquitination in the growth cone, and that the response to these cues requires a functional proteasome.
Myat, A. et al. Drosophila Nedd4, a ubiquitin ligase, is recruited by Commissureless to control cell surface levels of the Roundabout receptor. Neuron 35, 447–459 (2002).The authors show that ubiquitin-dependent mechanisms regulate the levels of the Robo receptor, and thereby allow the growth cone to change its responsiveness to the midline repellant Slit during axon pathfinding.
Kidd, T. et al. Dosage-sensitive and complementary functions of Roundabout and Commissureless control axon crossing of the CNS midline. Neuron 20, 25–33 (1998).
Kidd, T. et al. Slit is the midline repellent for the Robo receptor in Drosophila. Cell 96, 785–794 (1999).
Wolf, B. et al. Commissureless endocytosis is correlated with initiation of neuromuscular synaptogenesis. Development 125, 3853–3863 (1998).
Huang, Y. et al. Control of cell fate by a deubiquitinating enzyme encoded by the fat facets gene. Science 270, 1828–1831 (1995).
Wan, H. I. et al. Highwire regulates synaptic growth in Drosophila. Neuron 26, 313–329 (2000).
Joazeiro, C. A. & Weissman, A. M. RING finger proteins: mediators of ubiquitin ligase activity. Cell 102, 549–552 (2000).
Schaefer, A. M. et al. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron 26, 345–356 (2000).
Zhen, M. et al. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron 26, 331–343 (2000).
Guo, Q. et al. Identification of a large Myc-binding protein that contains RCC1-like repeats. Proc. Natl Acad. Sci. USA 95, 9172–9177 (1998).
Chen, X. et al. A specific protein substrate for a deubiquitinating enzyme: Liquid facets is the substrate of Fat facets. Genes Dev. 16, 289–294 (2002).
Craig, A. M. Activity and synaptic receptor targeting: the long view. Neuron 21, 459–462 (1998).
Terrell, J., Shih, S., Dunn, R. & Hicke, L. A function for monoubiquitination in the internalization of a G-protein coupled receptor. Mol. Cell 1, 193–202 (1998).
Hicke, L. Protein regulation by monoubiquitin. Nature Rev. Mol. Cell Biol. 2, 195–201 (2001).
Buttner, C. et al. Ubiquitination precedes internalization and proteolytic cleavage of plasma membrane-bound glycine receptors. J. Biol. Chem. 276, 42978–42985 (2001).
Bedford, F. K. et al. GABAA receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nature Neurosci. 4, 908–916 (2001).
Burbea, M. et al. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron 35, 107–120 (2002).The authors provide evidence that, in C. elegans , the ubiquitination of postsynaptic glutamate receptors regulates both synaptic strength and synapse number.
Wheeler, T. C. et al. Regulation of synaptophysin degradation by mammalian homologues of Seven in Absentia. J. Biol. Chem. 277, 10273–10282 (2002).
Abrams, T. W. Activity-dependent presynaptic facilitation: an associative mechanism in Aplysia. Cell. Mol. Neurobiol. 5,123–145 (1985).
Byrne, J. H. & Kandel, E. R. Presynaptic facilitation revisited: state and time dependence. J. Neurosci. 16, 425–435 (1996).
Chain, D. G. et al. Mechanisms for generating the autonomous cAMP-dependent protein kinase required for long-term facilitation in Aplysia. Neuron 22, 1–10 (1999).
Jiang, Y.-H. et al. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron 21, 799–811 (1998).Using a knockout approach, this paper shows that a ubiquitin ligase (E3) is crucial for long-term synaptic plasticity in vertebrates. The rationale for this study is provided by genetic evidence linking the E6-AP ubiquitin ligase to a human disorder called Angelman's syndrome (see references 49 and 50).
Kishino, T., Lalande, M. & Wagstaff, J. UBE3a/E6-AP mutations cause Angelman syndrome. Nature Genet. 15, 70–73 (1997).Together with reference 50 , this paper provides genetic evidence to link mutation in a ubiquitin ligase to a human disorder that is characterized by mental retardation.
Matsuura, T. et al. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nature Genet. 15, 74–77 (1997).
Lopez-Salon M. et al. The ubiquitin–proteasome cascade is required for mammalian long-term memory formation. Eur. J. Neurosci. 14, 1820–1826 (2001).
Abriel, H., Kamynina, E., Horisberger, J. D. & Staub, O. Regulation of the cardiac voltage-gated Na+ channel (H1) by the ubiquitin-protein ligase Nedd4. FEBS Lett. 466, 377–380 (2000).
Staub, O. et al. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J. 16, 6325–6336 (1997).
Long, X. & Griffith, L. C. Identification and characterization of a SUMO-1 conjugation system that modifies neuronal calcium/calmodulin-dependent protein kinase II in Drosophila melanogaster. J. Biol. Chem. 275, 40765–40776 (2000).
Mori, S., Heldin, C. H. & Claesson-Welsh, L. Ligand-induced polyubiquitination of the platelet-derived growth factor β-receptor. J. Biol. Chem. 267, 6429–6434 (1992).
Chen, H. et al. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature 394, 793–797 (1998).
Polo, S. et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature 416, 451–455 (2002).
Shih, S. C. et al. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nature Cell Biol. 4, 389–393 (2002).
Raiborg C. et al. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nature Cell Biol. 4, 394–398 (2002).
Katzmann, D. J., Babust, M. & Emr, S. D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I Cell 106, 145–155 (2001).
Orian, A. et al. SCFβ-TrCP ubiquitin ligase-mediated processing of NF-κB p105 requires phosphorylation of its C-terminus by IκB kinase. EMBO J. 19, 2580–2591 (2000).
Kaiser, P., Flick, K., Wittenberg, C. & Reed, S. I. Regulation of transcription by ubiquitination without proteolysis: Cdc34/SCFMet30-mediated inactivation of the transcription factor Met4. Cell 102, 303–314 (2000).
Salghetti, S. E., Caudy, A. A., Chenoweth, J. G. & Tansey, W. P. Regulation of transcriptional activation domain function by ubiquitin. Science 293, 1651–1653 (2001).
Conaway, R. C., Brower, C. S. & Conaway, J. W. Emerging roles of ubiquitin in transcription regulation. Science 296, 1254–1258 (2002).
Acknowledgements
This work was supported by grants from the Whitehall Foundation and the National Institute of Mental Health to A.N.H., and by grants from the Sloan, McKnight and Keck Foundations to A.D.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
FlyBase
GenBank
LocusLink
<i>Saccharomyces</i> Genome Database
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- EPIGENETIC
-
Describes a change in phenotype that is brought about by changes in the regulation of gene expression or changes in the function of gene products, rather than by a change in genotype. Examples of epigenetic events include DNA methylation, X-chromosome inactivation and embryonic pattern formation.
- LONG-TERM POTENTIATION
-
(LTP). An enduring increase in the amplitude of excitatory postsynaptic potentials as a result of high-frequency (tetanic) stimulation of afferent pathways. It is measured both as the amplitude of excitatory postsynaptic potentials and as the magnitude of the postsynaptic-cell population spike. LTP is most often studied in the hippocampus and is often considered to be the cellular basis of learning and memory in vertebrates.
- LONG-TERM FACILITATION
-
(LTF). A lasting increase in the strength of synapses between sensory and motor neurons in Aplysia. LTF is the cellular mechanism that underlies non-associative learning and memory. LTF results largely from presynaptic changes. It is similar to the LTP of the hippocampal mossy fibre pathway, but differs from LTP in other regions of the hippocampus, which are associative.
- HEAT SHOCK
-
A cellular response that is caused by exposure to a temperature that is higher than normal. The heat-shock proteins, many of which function as molecular chaperones, are synthesized in larger amounts under these conditions, and many of them are also crucial for cellular functions under non-stress conditions.
- BENDLESS
-
A Drosophila mutant in which synaptic connectivity is disrupted, leading to defects in the escape jump response. The bendless gene has been found to code for a ubiquitin-conjugating protein, and this mutant provided the first indication that ubiquitination is important for the wiring of the nervous system.
- RING-H2 DOMAIN
-
One of a class of protein domains that consist of two loops that are held together at their base by cysteine and histidine residues that complex two zinc ions. Proteins that contain domains of this type are known as RING-finger proteins.
- ANGELMAN'S SYNDROME
-
A genetic disorder that is caused by deletion or disruption of UBE3A (E6-AP). The symptoms of Angelman's syndrome include hyperactivity, ataxia, problems with speech and language, and an unusually happy demeanour.
- RETROGRADE AMNESIA
-
Loss of or inability to recall information that was previously stored in long-term memory.
- INHIBITORY AVOIDANCE
-
A learning paradigm in which an animal is taught to associate a particular location (such as a dark compartment in a maze) with an aversive stimulus (such as a foot shock).
Rights and permissions
About this article
Cite this article
Hegde, A., DiAntonio, A. Ubiquitin and the synapse. Nat Rev Neurosci 3, 854–861 (2002). https://doi.org/10.1038/nrn961
Issue Date:
DOI: https://doi.org/10.1038/nrn961
This article is cited by
-
USP39 is essential for mammalian epithelial morphogenesis through upregulation of planar cell polarity components
Communications Biology (2022)
-
Neuron-specific deficits of bioenergetic processes in the dorsolateral prefrontal cortex in schizophrenia
Molecular Psychiatry (2019)
-
Posttranslational Modifications Regulate the Postsynaptic Localization of PSD-95
Molecular Neurobiology (2017)
-
Association study of ubiquitin-specific peptidase 46 (USP46) with bipolar disorder and schizophrenia in a Japanese population
Journal of Human Genetics (2010)
-
Proteome analysis of lumbar spinal cord from rats submitted to peripheral lesion during neonatal period
Journal of Neural Transmission (2010)