Key Points
-
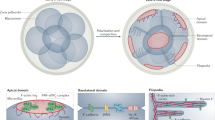
The sprouting and elongation of axons and dendrites forms the basis of correct neuronal connectivity and brain function. The initial sprouting of a neurite is a three step process — the original round shape of the cell is broken down to make a bud, the bud is transformed into a neurite, then the neurite is transformed into an axon or dendrite.
-
The basic engine that generates the force required for neurite extension is thought to be the actin cytoskeleton, although membrane addition and microtubules are also important for the maintenance of the elongated neurite, its polarity and its speed of growth.
-
The morphology and orientation of early neurites reflect the nature of the molecules in the surrounding environment. This review focuses on the integrin–laminin complex to illustrate the role of the external world in the initiation of neuronal differentiation.
-
Integrin receptor activation produces a membrane change that results in neurite protrusion, indicating that the making of a neurite might be determined by the presence of discrete microdomains or 'hot spots' on the plasma membrane.
-
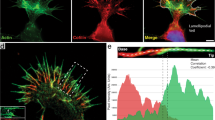
An early sign of the contact between the neuronal membrane and an extracellular ligand is a change in membrane appearance, from an even surface to one that is ornamented with veils (lamellipodia) and spikes (filopodia). This is preceded by the formation of thin membrane tethers that are mobilized from internal membrane stores. Localized addition of new membrane through the exocytotic pathway might account for the stabilization of lamellipodia and filopodia into real neurites.
-
Neurite protrusion requires the actin cytoskeleton, and high or low actin turnover seem to form the basis of neurite outgrowth or quiescence, respectively. Studies in fibroblasts and budding yeast have shown that actin-based motility is regulated by the Rho family of GTPases, and there is strong evidence that a similar mechanism operates in neurons.
-
Stimulation of certain receptors causes Rho proteins to undergo changes in activity, which lead to the formation of multimolecular complexes that can induce actin remodelling and cell movement. These complexes contain not only the Rhos and their regulatory proteins, but also actin-binding molecules and scaffold proteins, including the formins.
-
The microtubule cytoskeleton is another postulated target of Rho GTPases, and protrusion of microtubules into newly formed neurites seems to be required to sustain their development and elongation.
-
Mutations that affect neurite extension cause neurological diseases in humans that range from varying degrees of mental retardation to severe heterotopias. Understanding the secrets behind the conversion of a spherical neuronal-precursor cell into a fully differentiated neuron might lead to better approaches to treat these disorders.
Abstract
The sprouting of neurites, which will later become axons and dendrites, is an important event in early neuronal differentiation. Studies in living neurons indicate that neuritogenesis begins immediately after neuronal commitment, with the activation of membrane receptors by extracellular cues. These receptors activate intracellular cascades that trigger changes in the actin cytoskeleton, which promote the initial breakdown of symmetry. Then, through the regulation of gene transcription, and of microtubule and membrane dynamics, the newly formed neurite becomes stabilized. A key challenge is to define the molecular machinery that regulates the actin cytoskeleton during initial neurite sprouting. We propose that analysing the molecules involved in actin-dependent mechanisms in non-neuronal systems, such as budding yeast and migrating fibroblasts, could help to uncover the secrets of neuritogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mitchison, T. J. & Cramer, L. P. Actin-based cell motility and cell locomotion. Cell 84, 371–379 (1996).
Geiger, B., Bershadsky, A., Pankov, R. & Yamada, K. M. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nature Rev. Mol. Cell Biol. 2, 793–805 (2001).
Lambert de Rouvroit, C. & Goffinet, A. M. Neuronal migration. Mech. Dev. 105, 47–56 (2001).
de Curtis, I. Cell migration: GAPs between membrane traffic and the cytoskeleton. EMBO Rep. 2, 277–281 (2001).
Zheng, J. et al. Tensile regulation of axonal elongation and initiation. J. Neurosci. 11, 1117–1125 (1991).
Zakharenko, S. & Popov, S. Dynamics of axonal microtubules regulate the topology of new membrane insertion into the growing neurites. J. Cell Biol. 143, 1077–1086 (1998).
Lee, J., Ishiara, A. & Jacobson, K. How do cells move along surfaces? Trends Cell Biol. 3, 366–370 (1993).
Bray, D. The dynamics of growing axons. Membrane biophysics. Curr. Biol. 6, 241–243 (1996).
Bray, D. & Chapman, K. Analysis of microspike movements on the neuronal growth cone. J Neurosci. 5, 3204–3213 (1985).
Sheetz, M. P., Wayne, D. B. & Pearlman, A. L. Extension of filopodia by motor-dependent actin assembly. Cell Motil. Cytoskeleton 22, 160–169 (1992).
Isbister, C. M. & O'Connor, T. P. Filopodial adhesion does not predict growth cone steering events in vivo. J. Neurosci. 19, 2589–2600 (1999).
Puelles, L. & Privat, A. Do oculomotor neuroblasts migrate across the midline in the retal rat brain? Anat. Embryol. (Berl.) 150, 187–206 (1977).
Shoukimas, G. M. & Hinds, J. W. The development of the cerebral cortex in the embryonic mouse: an electron microscopic serial section analysis. J. Comp. Neurol. 179, 795–830 (1978).
Bourrat, F. & Sotelo, C. Migratory pathways and neuritic differentiation of inferior olivary neurons in the rat embryo. Axonal tracing study using the in vitro slab technique. Brain Res. 467, 19–37 (1988).
Rakic, P. Principles of neural cell migration. Experientia 46, 882–891 (1990).
Rakic, P. Neuron–glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus rhesus. J. Comp. Neurol. 141, 283–312 (1971).
Rakic, P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 145, 61–83 (1972).
Rakic, P. Neuronal migration and contact guidance in the primate telencephalon. Postgrad. Med. J. 54, 25–40 (1978).
Edmondson, J. C. & Hatten, M. E. Glial-guided granule neuron migration in vitro: a high-resolution time-lapse video microscopic study. J. Neurosci. 7, 1928–1934 (1987).
Hatten, M. E. The role of migration in central nervous system neuronal development. Curr. Opin. Neurobiol. 3, 38–44 (1993).
Gao, W. Q. & Hatten, M. E. Immortalizing oncogenes subvert the establishment of granule cell identity in developing cerebellum. Development 120, 1059–1070 (1994).
Hatten, M. E. Central nervous system neuronal migration. Annu. Rev. Neurosci. 22, 511–539 (1999).References 15–22 , together with reference 41 , provide a comprehensive outlook on neuronal migration, from morphological analysis to diseases that result from migration defects.
Nadarajah, B. & Parnavelas, J. G. Gap junction-mediated communication in the developing and adult cerebral cortex. Novartis Found. Symp. 219, 157–170 (1999).
Luskin, M. B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11, 173–189 (1993).
Denaxa, M., Chan, C. H., Schachner, M., Parnavelas, J. G. & Karagogeos, D. The adhesion molecule TAG-1 mediates the migration of cortical interneurons from the ganglionic eminence along the corticofugal fiber system. Development 128, 4635–4644 (2001).
Kaethner, R. J. & Stuermer, C. A. Dynamics of process formation during differentiation of tectal neurons in embryonic zebrafish. J. Neurobiol. 32, 627–639 (1997).
Dingwell, K. S., Holt, C. E. & Harris, W. A. The multiple decisions made by growth cones of RGCs as they navigate from the retina to the tectum in Xenopus embryos. J. Neurobiol. 44, 246–259 (2000).
Brittis, P. A., Meiri, K., Dent, E. & Silver, J. The earliest patterns of neuronal differentiation and migration in the mammalian central nervous system. Exp. Neurol. 134, 1–12 (1995).References 26–28 make an important contribution to our understanding of neurite formation and navigation in living specimens.
Craig, A. M. & Banker, G. Neuronal polarity. Annu. Rev. Neurosci. 17, 267–310 (1994).
Goslin, K. & Banker, G. Experimental observations on the development of polarity by hippocampal neurons in culture. J. Cell Biol. 108, 1507–1516 (1989).
Mason, C. A. & Wang, L. C. Growth cone form is behavior-specific and, consequently, position-specific along the retinal axon pathway. J. Neurosci. 17, 1086–1100 (1997).Together with reference 29 , this paper provides a thorough description of neuronal polarity development and growth cone behaviour in different types of neuronal systems both in vivo and in vitro.
Rauvala, H. & Peng, H. B. HB-GAM (heparin-binding growth-associated molecule) and heparin-type glycans in the development and plasticity of neuron-target contacts. Prog. Neurobiol. 52, 127–144 (1997).
Joester, A. & Faissner, A. The structure and function of tenascins in the nervous system. Matrix Biol. 20, 13–22 (2001).
Brose, K. & Tessier-Lavigne, M. Slit proteins: key regulators of axon guidance, axonal branching, and cell migration. Curr. Opin. Neurobiol. 10, 95–102 (2000).
Tucker, K. L., Meyer, M. & Barde, Y. A. Neurotrophins are required for nerve growth during development. Nature Neurosci. 4, 29–37 (2001).
Labelle, C. & Leclerc, N. Exogenous BDNF, NT-3 and NT-4 differentially regulate neurite outgrowth in cultured hippocampal neurons. Brain Res. Dev. Brain Res. 123, 1–11 (2000).
Mendell, L. M., Munson, J. B. & Arvanian, V. L. Neurotrophins and synaptic plasticity in the mammalian spinal cord. J. Physiol. (Lond.) 533, 91–97 (2001).
Hu, S. & Reichardt, L. F. From membrane to cytoskeleton: enabling a connection. Neuron 22, 419–422 (1999).
Lilienbaum, A., Reszka, A. A., Horwitz, A. F. & Holt, C. E. Chimeric integrins expressed in retinal ganglion cells impair process outgrowth in vivo. Mol. Cell. Neurosci. 6, 139–152 (1995).
Baum, P. D. & Garriga, G. Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron 19, 51–62 (1997).In references 39 and 40 , the importance of integrins in neuritogenesis is studied in two different biological systems.
Gleeson, J. G. & Walsh, C. A. Neuronal migration disorders: from genetic diseases to developmental mechanisms. Trends Neurosci. 23, 352–359 (2000).
Gomez, T. M., Robles, E., Poo, M. & Spitzer, N. C. Filopodial calcium transients promote substrate-dependent growth cone turning. Science 291, 1983–1987 (2001).
Spitzer, N. C. Activity-dependent neuronal differentiation prior to synapse formation: the functions of calcium transients. J. Physiol. (Paris) 96, 73–80 (2002).
Chang, S. & De Camilli, P. Glutamate regulates actin-based motility in axonal filopodia. Nature Neurosci. 4, 787–793 (2001).
Lautermilch, N. J. & Spitzer, N. C. Regulation of calcineurin by growth cone calcium waves controls neurite extension. J. Neurosci. 20, 315–325 (2000).
Burkin, D. J., Kim, J. E., Gu, M. & Kaufman, S. J. Laminin and α7β1 integrin regulate agrin-induced clustering of acetylcholine receptors. J. Cell Sci. 113, 2877–2886 (2000).
Wodarz, A., Ramrath, A., Kuchinke, U. & Knust, E. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature 402, 544–547 (1999).
Garner, C. C., Nash, J. & Huganir, R. L. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 10, 274–280 (2000).
Harris, B. Z. & Lim, W. A. Mechanism and role of PDZ domains in signaling complex assembly. J. Cell Sci. 114, 3219–3231 (2001).
Cayouette, M., Whitmore, A. V., Jeffery, G. & Raff, M. Asymmetric segregation of Numb in retinal development and the influence of the pigmented epithelium. J. Neurosci. 21, 5643–5651 (2001).This work, together with that of reference 47 , paved the way for studying whether or not mammalian neurons are 'primed' for the site of sprouting before migration.
Condeelis, J. Life at the leading edge: the formation of cell protrusions. Annu. Rev. Cell Biol. 9, 411–444 (1993).
Pantaloni, D., Le Clainche, C. & Carlier, M. F. Mechanism of actin-based motility. Science 292, 1502–1506 (2001).
Raucher, D. & Sheetz, M. P. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J. Cell Biol. 148, 127–136 (2000).
Raucher, D. & Sheetz, M. P. Characteristics of a membrane reservoir buffering membrane tension. Biophys. J. 77, 1992–2002 (1999).
Morris, C. E. & Homann, U. Cell surface area regulation and membrane tension. J. Membr. Biol. 179, 79–102 (2001).References 53–55 provide experimental evidence for how membrane tension can regulate surface deformation in neurons and thus influence neuritogenesis.
Holt, C. E. A single-cell analysis of early retinal ganglion cell differentiation in Xenopus: from soma to axon tip. J. Neurosci. 9, 3123–3145 (1989).
Tang, B. L. Protein trafficking mechanisms associated with neurite outgrowth and polarized sorting in neurons. J. Neurochem. 79, 923–930 (2001).
Mattson, M. P. Establishment and plasticity of neuronal polarity. J. Neurosci. Res. 57, 577–589 (1999).
Futerman, A. H. & Banker, G. A. The economics of neurite outgrowth — the addition of new membrane to growing axons. Trends Neurosci. 19, 144–149 (1996).
Bradke, F. & Dotti, C. G. Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron 19, 1175–1186 (1997).
Smith, S. J. Neuronal cytomechanics: the actin-based motility of growth cones. Science 242, 708–715 (1988).
Pollard, T. D. Reflections on a quarter century of research on contractile systems. Trends Biochem. Sci. 25, 607–611 (2000).
Herring, T. L., Cohan, C. S., Welnhofer, E. A., Mills, L. R. & Morris, C. E. F-actin at newly invaginated membrane in neurons: implications for surface area regulation. J. Membr. Biol. 171, 151–169 (1999).
Goldberg, D. J. & Burmeister, D. W. Looking into growth cones. Trends Neurosci. 12, 503–506 (1989).
Zheng, J. Q., Felder, M., Connor, J. A. & Poo, M. M. Turning of nerve growth cones induced by neurotransmitters. Nature 368, 140–144 (1994).
Bridgman, P. C. & Dailey, M. E. The organization of myosin and actin in rapid frozen nerve growth cones. J. Cell Biol. 108, 95–109 (1989).
Letourneau, P. C. Differences in the organization of actin in the growth cones compared with the neurites of cultured neurons from chick embryos. J. Cell Biol. 97, 963–973 (1983).
Forscher, P. & Smith, S. J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 107, 1505–1516 (1988).
Bradke, F. & Dotti, C. G. The role of local actin instability in axon formation. Science 283, 1931–1934 (1999).
Ruthel, G. & Banker, G. Role of moving growth cone-like 'wave' structures in the outgrowth of cultured hippocampal axons and dendrites. J. Neurobiol. 39, 97–106 (1999).
Ruthel, G. & Hollenbeck, P. J. Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons. J. Neurosci. 20, 2266–2274 (2000).Together, references 68–71 provide experimental evidence for how actin dynamics are involved in growth cone motility and process formation.
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998).
Van Aelst, L. & D'Souza-Schorey, C. Rho GTPases and signaling networks. Genes Dev. 11, 2295–2322 (1997).
Ridley, A. J. & Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399 (1992).
Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D. & Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410 (1992).
Nobes, C. D. & Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62 (1995).
Kozma, R., Ahmed, S., Best, A. & Lim, L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 15, 1942–1952 (1995).
Olson, M. F., Pasteris, N. G., Gorski, J. L. & Hall, A. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr. Biol. 6, 1628–1633 (1996).
Michiels, F. et al. Regulated membrane localization of Tiam1, mediated by the NH2-terminal pleckstrin homology domain, is required for Rac-dependent membrane ruffling and c-Jun NH2-terminal kinase activation. J. Cell Biol. 137, 387–398 (1997).
Michelitch, M. & Chant, J. A mechanism of Bud1p GTPase action suggested by mutational analysis and immunolocalization. Curr. Biol. 6, 446–454 (1996).
Park, H. O., Chant, J. & Herskowitz, I. BUD2 encodes a GTPase-activating protein for Bud1/Rsr1 necessary for proper bud-site selection in yeast. Nature 365, 269–274 (1993).
Johnson, D. I. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63, 54–105 (1999).
Schmidt, A. & Hall, M. N. Signaling to the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 14, 305–338 (1998).
Tanaka, K. & Takai, Y. Control of reorganization of the actin cytoskeleton by Rho family small GTP-binding proteins in yeast. Curr. Opin. Cell Biol. 10, 112–116 (1998).
Jalink, K., Eichholtz, T., Postma, F. R., van Corven, E. J. & Moolenaar, W. H. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 4, 247–255 (1993).
Jalink, K. et al. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol. 126, 801–10 (1994).
Nishiki, T. et al. ADP-ribosylation of the rho/rac proteins induces growth inhibition, neurite outgrowth and acetylcholine esterase in cultured PC-12 cells. Biochem. Biophys. Res. Commun. 167, 265–272 (1990).
Aktories, K. Rho proteins: targets for bacterial toxins. Trends Microbiol. 5, 282–288 (1997).
Ottlinger, M. E. & Lin, S. Clostridium difficile toxin B induces reorganization of actin, vinculin, and talin in cultured cells. Exp. Cell Res. 174, 215–229 (1988).
Luo, L., Liao, Y. J., Jan, L. Y. & Jan, Y. N. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8, 1787–1802 (1994).
Luo, L. Rho GTPases in neuronal morphogenesis. Nature Rev. Neurosci. 1, 173–180 (2000).Together with references 72 and 73 , this paper offers comprehensive revisions on the role and regulation of Rho GTPases in a wide spectrum of biological systems.
Kuhn, T. B., Brown, M. D. & Bamburg, J. R. Rac1-dependent actin filament organization in growth cones is necessary for β1-integrin-mediated advance but not for growth on poly-d-lysine. J. Neurobiol. 37, 524–540 (1998).
Kaufmann, N., Wills, Z. P. & Van Vactor, D. Drosophila Rac1 controls motor axon guidance. Development 125, 453–461 (1998).
Ruchhoeft, M. L., Ohnuma, S., McNeill, L., Holt, C. E. & Harris, W. A. The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J. Neurosci. 19, 8454–8463 (1999).
Threadgill, R., Bobb, K. & Ghosh, A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron 19, 625–634 (1997).
Luo, L. et al. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature 379, 837–840 (1996).
Li, Z., Van Aelst, L. & Cline, H. T. Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nature Neurosci. 3, 217–225 (2000).
Nakayama, A. Y., Harms, M. B. & Luo, L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. 20, 5329–5338 (2000).
Wong, W. T., Faulkner-Jones, B. E., Sanes, J. R. & Wong, R. O. Rapid dendritic remodeling in the developing retina: dependence on neurotransmission and reciprocal regulation by Rac and Rho. J. Neurosci. 20, 5024–5036 (2000).
Harris, K. M. Structure, development, and plasticity of dendritic spines. Curr. Opin. Neurobiol. 9, 343–348 (1999).
Wasserman, S. FH proteins as cytoskeletal organizers. Trends Cell Biol. 8, 111–115 (1998).
Frazier, J. A. & Field, C. M. Actin cytoskeleton: are FH proteins local organizers? Curr. Biol. 7, R414–R417 (1997).
Sheu, Y. J., Santos, B., Fortin, N., Costigan, C. & Snyder, M. Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol. Cell. Biol. 18, 4053–4069 (1998).
Fujiwara, T. et al. Rho1p–Bni1p–Spa2p interactions: implication in localization of Bni1p at the bud site and regulation of the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell 9, 1221–1233 (1998).
Imamura, H. et al. Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16, 2745–2755 (1997).
Pruyne, D. & Bretscher, A. Polarization of cell growth in yeast. J. Cell Sci. 113, 571–585 (2000).
Evangelista, M., Pruyne, D., Amberg, D. C., Boone, C. & Bretscher, A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nature Cell Biol. 4, 32–41 (2002).
Sagot, I., Klee, S. K. & Pellman, D. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nature Cell Biol. 4, 42–50 (2002).Articles 107 and 108 are recent influential contributions on the role of formins in bud formation; they highlight the potential involvement of this group of proteins in the budding of other biological spheres (for example, neurons).
Borisy, G. G. & Svitkina, T. M. Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12, 104–112 (2000).
Cooper, J. A. & Schafer, D. A. Control of actin assembly and disassembly at filament ends. Curr. Opin. Cell Biol. 12, 97–103 (2000).
Carlsson, L., Nystrom, L. E., Sundkvist, I., Markey, F. & Lindberg, U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J. Mol. Biol. 115, 465–483 (1977).
Di Nardo, A., Gareus, R., Kwiatkowski, D. & Witke, W. Alternative splicing of the mouse profilin II gene generates functionally different profilin isoforms. J. Cell Sci. 113, 3795–3803 (2000).
Hu, E., Chen, Z., Fredrickson, T. & Zhu, Y. Molecular cloning and characterization of profilin-3: a novel cytoskeleton-associated gene expressed in rat kidney and testes. Exp. Nephrol. 9, 265–274 (2001).
Haarer, B. K. et al. Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J. Cell Biol. 110, 105–114 (1990).
Buss, F., Temm-Grove, C., Henning, S. & Jockusch, B. M. Distribution of profilin in fibroblasts correlates with the presence of highly dynamic actin filaments. Cell Motil. Cytoskeleton 22, 51–61 (1992).
Suetsugu, S., Miki, H. & Takenawa, T. Distinct roles of profilin in cell morphological changes: microspikes, membrane ruffles, stress fibers, and cytokinesis. FEBS Lett. 457, 470–474 (1999).References 115 and 116 describe the localization and role of profilin in different actin-driven morphological events in fibroblasts.
Maciver, S. K. How ADF/cofilin depolymerizes actin filaments. Curr. Opin. Cell Biol. 10, 140–144 (1998).
Mulholland, J. et al. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J. Cell Biol. 125, 381–391 (1994).
Moon, A. L., Janmey, P. A., Louie, K. A. & Drubin, D. G. Cofilin is an essential component of the yeast cortical cytoskeleton. J. Cell Biol. 120, 421–435 (1993).
Lappalainen, P. & Drubin, D. G. Cofilin promotes rapid actin filament turnover in vivo. Nature 388, 78–82 (1997).
Yonezawa, N. et al. Distribution among tissues and intracellular localization of cofilin, a 21kDa actin-binding protein. Cell Struct. Funct. 12, 443–452 (1987).
Higgs, H. N. & Pollard, T. D. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70, 649–676 (2001).
Machesky, L. M. et al. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl Acad. Sci. USA 96, 3739–3744 (1999).
Higgs, H. N. & Pollard, T. D. Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins. J. Biol. Chem. 274, 32531–32534 (1999).
Lechler, T., Shevchenko, A. & Li, R. Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. J. Cell Biol. 148, 363–373 (2000).
Naqvi, S. N., Zahn, R., Mitchell, D. A., Stevenson, B. J. & Munn, A. L. The WASp homologue Las17p functions with the WIP homologue End5p/verprolin and is essential for endocytosis in yeast. Curr. Biol. 8, 959–962 (1998).
Winter, D., Podtelejnikov, A. V., Mann, M. & Li, R. The complex containing actin-related proteins Arp2 and Arp3 is required for the motility and integrity of yeast actin patches. Curr. Biol. 7, 519–529 (1997).
Machesky, L. M. & Insall, R. H. Scar1 and the related Wiskott–Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8, 1347–1356 (1998).
Castrillon, D. H. & Wasserman, S. A. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development 120, 3367–3377 (1994).
Watanabe, N. et al. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 16, 3044–3056 (1997).
Watanabe, N., Kato, T., Fujita, A., Ishizaki, T. & Narumiya, S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nature Cell Biol. 1, 136–143 (1999).
Krebs, A., Rothkegel, M., Klar, M. & Jockusch, B. M. Characterization of functional domains of mDia1, a link between the small GTPase Rho and the actin cytoskeleton. J. Cell Sci. 114, 3663–3672 (2001).
Verheyen, E. M. & Cooley, L. Profilin mutations disrupt multiple actin-dependent processes during Drosophila development. Development 120, 717–728 (1994).
Wills, Z., Marr, L., Zinn, K., Goodman, C. S. & Van Vactor, D. Profilin and the Abl tyrosine kinase are required for motor axon outgrowth in the Drosophila embryo. Neuron 22, 291–299 (1999).
Lambrechts, A. et al. Profilin II is alternatively spliced, resulting in profilin isoforms that are differentially expressed and have distinct biochemical properties. Mol. Cell. Biol. 20, 8209–8219 (2000).
Bito, H. et al. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron 26, 431–441 (2000).This work implicates RhoA and ROCK in neurite initiation, and indicates that RhoA inhibition favours such an event.
Jasper, H. et al. The genomic response of the Drosophila embryo to JNK signaling. Dev. Cell 1, 579–586 (2001).
Smith, C. L. Cytoskeletal movements and substrate interactions during initiation of neurite outgrowth by sympathetic neurons in vitro. J. Neurosci. 14, 384–398 (1994).
Smith, C. L. The initiation of neurite outgrowth by sympathetic neurons grown in vitro does not depend on assembly of microtubules. J. Cell Biol. 127, 1407–1418 (1994).
Tang, D. & Goldberg, D. J. Bundling of microtubules in the growth cone induced by laminin. Mol. Cell. Neurosci. 15, 303–313 (2000).
Ahmad, F. J. et al. Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nature Cell Biol. 2, 276–280 (2000).
Zhou, F. Q., Waterman-Storer, C. M. & Cohan, C. S. Focal loss of actin bundles causes microtubule redistribution and growth cone turning. J. Cell Biol. 157, 839–849 (2002).
Waterman-Storer, C. M., Worthylake, R. A., Liu, B. P., Burridge, K. & Salmon, E. D. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nature Cell Biol. 1, 45–50 (1999).
Wittmann, T. & Waterman-Storer, C. M. Cell motility: can Rho GTPases and microtubules point the way? J. Cell Sci. 114, 3795–3803 (2001).An important revision in which the interaction between actin and microtubules is highlighted in the context of the role of Rho GTPases in regulating the microtubule cytoskeleton during cell migration.
Dotti, C. G., Sullivan, C. A. & Banker, G. A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8, 1454–1468 (1988).
Diaz, H., Lorenzo, A., Carrer, H. F. & Caceres, A. Time lapse study of neurite growth in hypothalamic dissociated neurons in culture: sex differences and estrogen effects. J. Neurosci. Res. 33, 266–281 (1992).
Ferreira, A. & Caceres, A. The expression of acetylated microtubules during axonal and dendritic growth in cerebellar macroneurons which develop in vitro. Brain Res. Dev. Brain Res. 49, 205–213 (1989).
He, Z., Wang, K. C., Koprivica, V., Ming, G. & Song, H. J. Knowing how to navigate: mechanisms of semaphorin signaling in the nervous system. Sci STKE 2002, RE1 (2002).
Liu, B. P. & Strittmatter, S. M. Semaphorin-mediated axonal guidance via Rho-related G proteins. Curr. Opin. Cell Biol. 13, 619–626 (2001).
Wilkinson, D. G. Multiple roles of EPH receptors and ephrins in neural development. Nature Rev. Neurosci. 2, 155–164 (2001).
Klein, R. Excitatory Eph receptors and adhesive ephrin ligands. Curr. Opin. Cell Biol. 13, 196–203 (2001).
Esch, T., Lemmon, V. & Banker, G. Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J. Neurosci. 19, 6417–6426 (1999).
Lein, P., Johnson, M., Guo, X., Rueger, D. & Higgins, D. Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron 15, 597–605 (1995).
Lafont, F. & Prochiantz, A. Region-specific neuroastroglial interactions in neuronal morphogenesis and polarity: from homeogenic induction to cellular cytomechanics. Perspect. Dev. Neurobiol. 2, 259–268 (1994).
Acknowledgements
We thank all the members of the laboratory for their contribution to this work through helpful suggestions and insightful comments on the manuscript. J.S.S. is supported by an FCT/PRAXIS XXI scholarship (Portuguese Ministry of Science and Technology).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
FlyBase
LocusLink
<i>Saccharomyces</i> Genome Database
WormBase
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- LAMELLIPODIUM
-
A sheet-like extension at the edge of cells that contains a crosslinked F-actin meshwork and is often associated with cell migration.
- FILOPODIUM
-
A long, thin protrusion at the periphery of cells and growth cones that is composed of F-actin bundles.
- LATERAL GANGLIONIC EMINENCE
-
A region of the embryonic brain that gives rise to the striatum. It is also the source of a population of cells that migrate to populate the cerebral cortex and the olfactory bulb.
- DII
-
DiI is a lipophilic carbocyanine dye that emits an intense fluorescence when incorporated into cell membranes. It is commonly used to track cell migration, or for the retrograde or anterograde tracing of axons. It can be used on both live and fixed tissue.
- HETEROTOPIA
-
In general, this terms refers to the displacement of neuronal cell bodies into the white matter.
- PDZ DOMAIN
-
A peptide-binding domain that is important for the organization of membrane proteins, particularly at cell–cell junctions, including synapses. It can bind to the carboxyl termini of proteins or can form dimers with other PDZ domains. PDZ domains are named after the proteins in which these sequence motifs were originally identified (PSD95, Discs large, zona occludens 1).
- DOMINANT NEGATIVE
-
Describes a mutant molecule that can form a heteromeric complex with the normal molecule, knocking out the activity of the entire complex.
- STRESS FIBRES
-
Bundles of microfilaments and other proteins that are commonly found on migrating cells. They are contractile and can be anchored to a focal adhesion.
- FOCAL ADHESION
-
An area of close apposition between the membrane of a cell and the surface along which the cell is moving.
- SH DOMAINS
-
Src-homology domains are involved in interactions with phosphorylated tyrosine residues on other proteins (SH2 domains) or with proline-rich sections of other proteins (SH3 domains).
- ADP RIBOSYLATION
-
The transfer, through an ADP ribosyltransferase, of one or more ADP-ribosyl groups from NAD+ to a protein.
- PC12 CELLS
-
Neuron-like cells that are derived from a malignant neural crest tumour (a phaeochromocytoma).
- N1E-115 CELLS
-
An adrenergic cell line derived from a mouse neuroblastoma that is commonly used in studies of tumorigenicity.
- TOXIN B
-
A glucosyltransferase that inhibits members of the Rho family of GTPases. It is produced by Clostridium difficile, the causative agent of pseudomembranous colitis.
- POINTED END
-
The structural polarity of filamentous actin was defined by decoration with myosin fragments. This procedure resulted in an arrowhead appearance of the actin filament, with a 'barbed' end and a 'pointed' end. The addition of actin monomers is more favoured at the barbed end than at the pointed end.
- CORTICAL MESHWORK
-
A submembranous layer of microfilaments anchored to the cell membrane that contributes to the mechanical properties of the cell surface and restricts the access of cytoplasmic vesicles to the plasma membrane.
- SWISS 3T3 CELLS
-
An immortal line of fibroblast-like cells that was established from embryos of Swiss mice (which are not an inbred stock). They form confluent monolayers, and their mobility is highly inhibited by contact.
- CYTOKINESIS
-
The final event in the cell-division cycle. Its completion results in the irreversible partition of a mother cell into two daughter cells. It involves cytoplasmic division, driven by an actin-based constriction of the contractile ring. It is worthwhile comparing cytokinesis with mitosis — the process of nuclear division.
- RBD DOMAIN
-
Rho-GTP-binding domain. The RBD of the mammalian diaphanous proteins binds only activated RhoA–C, whereas diaphanous proteins of budding yeast interact with Cdc42 as well. The binding of activated Rho to the RBD of diaphanous proteins stimulates actin reorganization.
- CIID DOMAIN
-
C-terminal intramolecular domain of diaphanous proteins. The RBD domain complexes with CIID in a species-restricted manner to autoregulate the activity of diaphanous proteins.
Rights and permissions
About this article
Cite this article
Da Silva, J., Dotti, C. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci 3, 694–704 (2002). https://doi.org/10.1038/nrn918
Issue Date:
DOI: https://doi.org/10.1038/nrn918
This article is cited by
-
The cytoplasmic localization of ADNP through 14-3-3 promotes sex-dependent neuronal morphogenesis, cortical connectivity, and calcium signaling
Molecular Psychiatry (2023)
-
Mitochondrial, cell cycle control and neuritogenesis alterations in an iPSC-based neurodevelopmental model for schizophrenia
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Navigating the Landscape of MANF Research: A Scientometric Journey with CiteSpace Analysis
Cellular and Molecular Neurobiology (2023)
-
Single-cell transcriptomic analysis reveals the adverse effects of cadmium on the trajectory of neuronal maturation
Cell Biology and Toxicology (2023)
-
TTC3-Mediated Protein Quality Control, A Potential Mechanism for Cognitive Impairment
Cellular and Molecular Neurobiology (2022)